Indacaterol provides 24-hour bronchodilation in COPD: a placebo-controlled blinded comparison with tiotropium
- PMID: 20920365
- PMCID: PMC2964613
- DOI: 10.1186/1465-9921-11-135
Indacaterol provides 24-hour bronchodilation in COPD: a placebo-controlled blinded comparison with tiotropium
Abstract
Background: Indacaterol is a novel, inhaled, once-daily, ultra-long-acting β2-agonist for the treatment of chronic obstructive pulmonary disease (COPD). This randomized, double-blind study compared the bronchodilator efficacy of indacaterol with that of placebo and tiotropium in patients with moderate-to-severe COPD.
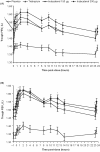
Methods: In an incomplete-block, multi-dose, three-period, crossover design, patients received three of the following four treatments: indacaterol 150 μg, indacaterol 300 μg, tiotropium 18 μg and placebo, each once-daily for 14 days. Each treatment period was separated by a 14-day washout. Study drug was supplied daily by blinded, third party study personnel to maintain blinding of patients and investigators. The primary efficacy variable was trough forced expiratory volume in one second (FEV1) at 24 h post-dose after 14 days. The study was powered to demonstrate non-inferiority of indacaterol to tiotropium for this variable.
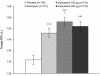
Results: A total of 169 patients were randomized (mean age 65 years); 153 (90.5%) completed. Trough FEV1 after 14 days with indacaterol 150 μg and 300 μg was statistically and clinically superior to placebo, with differences (95% CI) of 170 (120-220) and 150 (100-200) mL respectively (both p < 0.001). For this endpoint, both doses of indacaterol not only met the criterion for non-inferiority compared with tiotropium, but also achieved numerically higher values, with differences versus tiotropium of 40 and 30 mL for indacaterol 150 and 300 μg, respectively. At 5 min post-dose on Day 1, the mean FEV1 for both indacaterol doses was significantly higher than placebo (by 120 and 130 mL for indacaterol 150 and 300 μg, respectively; p < 0.001) and tiotropium (by 80 mL for both doses; p < 0.001). Adverse events were reported by similar proportions of patients: 31.4%, 29.5%, 28.3% and 28.5% for indacaterol 150 μg and 300 μg, tiotropium and placebo treatments, respectively.
Conclusions: Once-daily indacaterol provided clinically and statistically significant 24-h bronchodilation. Indacaterol was at least as effective as tiotropium, with a faster onset of action (within 5 min) on the first day of dosing. Indacaterol should prove useful in patients with moderate-to-severe COPD, for whom treatment with one or more classes of long-acting bronchodilator is recommended.
Trial registration: ClinicalTrials.gov: NCT00615459, EudraCT number: 2007-004071-19.
Figures
References
-
- global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2009. http://www.goldcopd.org Accessed: 01.03.2010. - PubMed
-
- Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) Eur Respir J. 1997;10:815–821. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

