A trial of clarithromycin for the treatment of suboptimally controlled asthma
- PMID: 20920764
- PMCID: PMC2950827
- DOI: 10.1016/j.jaci.2010.07.024
A trial of clarithromycin for the treatment of suboptimally controlled asthma
Abstract
Background: PCR studies have demonstrated evidence of Mycoplasma pneumoniae and Chlamydophila pneumoniae in the lower airways of patients with asthma.
Objective: To test the hypothesis that clarithromycin would improve asthma control in individuals with mild-to-moderate persistent asthma that was not well controlled despite treatment with low-dose inhaled corticosteroids.
Methods: Adults with an Asthma Control Questionnaire score ≥1.5 after a 4-week period of treatment with fluticasone propionate were entered into a PCR-stratified randomized, controlled trial to evaluate the effect of 16 weeks of either clarithromycin or placebo, added to fluticasone, on asthma control in individuals with or without lower airway PCR evidence of M pneumoniae or C pneumoniae.
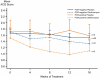
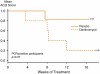
Results: A total of 92 participants were randomized. Twelve (13%) subjects demonstrated PCR evidence of M pneumoniae or C pneumoniae in endobronchial biopsies; 80 were PCR-negative for both organisms. In PCR-positive participants, clarithromycin yielded a 0.4 ± 0.4 unit improvement in the Asthma Control Questionnaire score, with a 0.1 ± 0.3 unit improvement in those allocated to placebo. This between-group difference of 0.3 ± 0.5 (P = .6) was neither clinically nor statistically significant. In PCR-negative participants, a nonsignificant between-group difference of 0.2 ± 0.2 units (P = .3) was observed. Clarithromycin did not improve lung function or airway inflammation but did improve airway hyperresponsiveness, increasing the methacholine PC(20) by 1.2 ± 0.5 doubling doses (P = .02) in the study population.
Conclusion: Adding clarithromycin to fluticasone in adults with mild-to-moderate persistent asthma that was suboptimally controlled by low-dose inhaled corticosteroids alone did not further improve asthma control. Although there was an improvement in airway hyperresponsiveness with clarithromycin, this benefit was not accompanied by improvements in other secondary outcomes.
Trial registration: ClinicalTrials.gov NCT00318708.
Copyright © 2010 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures

References
-
- Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007 Oct 11;357(15):1487–95. - PubMed
-
- Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001 Apr;107(4):595–601. - PubMed
-
- Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991;266(2):225–30. - PubMed
-
- Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. - PubMed
-
- Kraft M, Adler KB, Ingram JL, Crews AL, Atkinson TP, Cairns CB, et al. Mycoplasma pneumoniae induces airway epithelial cell expression of MUC5AC in asthma. Eur Respir J. 2008 Jan;31(1):43–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL074227/HL/NHLBI NIH HHS/United States
- K23 HL04385/HL/NHLBI NIH HHS/United States
- UL1-RR025011/RR/NCRR NIH HHS/United States
- U10 HL074212/HL/NHLBI NIH HHS/United States
- U10 HL074208/HL/NHLBI NIH HHS/United States
- UL1 RR025011/RR/NCRR NIH HHS/United States
- U10 HL074204/HL/NHLBI NIH HHS/United States
- K23 HL004385/HL/NHLBI NIH HHS/United States
- U10 HL074225/HL/NHLBI NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- U10 HL074073/HL/NHLBI NIH HHS/United States
- U10 HL074231/HL/NHLBI NIH HHS/United States
- U10 HL074206/HL/NHLBI NIH HHS/United States
- U10 HL074218/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

