Regulation of tau pathology by the microglial fractalkine receptor
- PMID: 20920788
- PMCID: PMC2950825
- DOI: 10.1016/j.neuron.2010.08.023
Regulation of tau pathology by the microglial fractalkine receptor
Abstract
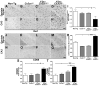
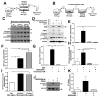
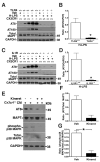
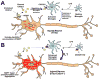
Aggregates of the hyperphosphorylated microtubule-associated protein tau (MAPT) are an invariant neuropathological feature of tauopathies. Here, we show that microglial neuroinflammation promotes MAPT phosphorylation and aggregation. First, lipopolysaccharide-induced microglial activation promotes hyperphosphorylation of endogenous mouse MAPT in nontransgenic mice that is further enhanced in mice lacking the microglial-specific fractalkine receptor (CX3CR1) and is dependent upon functional toll-like receptor 4 and interleukin-1 (IL-1) receptors. Second, humanized MAPT transgenic mice lacking CX3CR1 exhibited enhanced MAPT phosphorylation and aggregation as well as behavioral impairments that correlated with increased levels of active p38 MAPK. Third, in vitro experiments demonstrate that microglial activation elevates the level of active p38 MAPK and enhances MAPT hyperphosphorylation within neurons that can be blocked by administration of an interleukin-1 receptor antagonist and a specific p38 MAPK inhibitor. Taken together, our results suggest that CX3CR1 and IL-1/p38 MAPK may serve as novel therapeutic targets for human tauopathies.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. - PubMed
-
- Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1:213–216. - PubMed
-
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

