Sds22 regulates aurora B activity and microtubule-kinetochore interactions at mitosis
- PMID: 20921135
- PMCID: PMC2953433
- DOI: 10.1083/jcb.200912046
Sds22 regulates aurora B activity and microtubule-kinetochore interactions at mitosis
Abstract
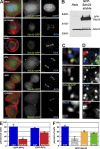
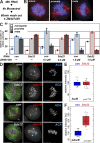
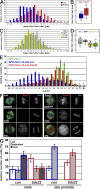
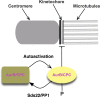
We have studied Sds22, a conserved regulator of protein phosphatase 1 (PP1) activity, and determined its role in modulating the activity of aurora B kinase and kinetochore-microtubule interactions. Sds22 is required for proper progression through mitosis and localization of PP1 to mitotic kinetochores. Depletion of Sds22 increases aurora B T-loop phosphorylation and the rate of recovery from monastrol arrest. Phospho-aurora B accumulates at kinetochores in Sds22-depleted cells juxtaposed to critical kinetochore substrates. Sds22 modulates sister kinetochore distance and the interaction between Hec1 and the microtubule lattice and, thus, the activation of the spindle assembly checkpoint. These results demonstrate that Sds22 specifically defines PP1 function and localization in mitosis. Sds22 regulates PP1 targeting to the kinetochore, accumulation of phospho-aurora B, and force generation at the kinetochore-microtubule interface.
Figures



Similar articles
-
Inhibitor-3 ensures bipolar mitotic spindle attachment by limiting association of SDS22 with kinetochore-bound protein phosphatase-1.EMBO J. 2014 Nov 18;33(22):2704-20. doi: 10.15252/embj.201489054. Epub 2014 Oct 8. EMBO J. 2014. PMID: 25298395 Free PMC article.
-
Sds22 and Repo-Man stabilize chromosome segregation by counteracting Aurora B on anaphase kinetochores.J Cell Biol. 2012 Jul 23;198(2):173-83. doi: 10.1083/jcb.201112112. Epub 2012 Jul 16. J Cell Biol. 2012. PMID: 22801782 Free PMC article.
-
Phosphorylation of microtubule-binding protein Hec1 by mitotic kinase Aurora B specifies spindle checkpoint kinase Mps1 signaling at the kinetochore.J Biol Chem. 2013 Dec 13;288(50):36149-59. doi: 10.1074/jbc.M113.507970. Epub 2013 Nov 1. J Biol Chem. 2013. PMID: 24187132 Free PMC article.
-
Spindle checkpoint silencing: PP1 tips the balance.Curr Biol. 2011 Nov 8;21(21):R898-903. doi: 10.1016/j.cub.2011.08.063. Curr Biol. 2011. PMID: 22075433 Review.
-
Regulation of kinetochore-microtubule attachments by Aurora B kinase.Biochem Soc Trans. 2009 Oct;37(Pt 5):976-80. doi: 10.1042/BST0370976. Biochem Soc Trans. 2009. PMID: 19754435 Review.
Cited by
-
Suppressors of ipl1-2 in components of a Glc7 phosphatase complex, Cdc48 AAA ATPase, TORC1, and the kinetochore.G3 (Bethesda). 2012 Dec;2(12):1687-701. doi: 10.1534/g3.112.003814. Epub 2012 Dec 1. G3 (Bethesda). 2012. PMID: 23275890 Free PMC article.
-
Delineating the contribution of Spc105-bound PP1 to spindle checkpoint silencing and kinetochore microtubule attachment regulation.J Cell Biol. 2019 Dec 2;218(12):3926-3942. doi: 10.1083/jcb.201810172. Epub 2019 Oct 24. J Cell Biol. 2019. PMID: 31649151 Free PMC article.
-
Measuring NDC80 binding reveals the molecular basis of tension-dependent kinetochore-microtubule attachments.Elife. 2018 Jul 25;7:e36392. doi: 10.7554/eLife.36392. Elife. 2018. PMID: 30044223 Free PMC article.
-
The Role of Mitotic Kinases and the RZZ Complex in Kinetochore-Microtubule Attachments: Doing the Right Link.Front Cell Dev Biol. 2022 Jan 28;10:787294. doi: 10.3389/fcell.2022.787294. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35155423 Free PMC article. Review.
-
Photobody Detection Using Immunofluorescence and Super-Resolution Imaging in Arabidopsis.Methods Mol Biol. 2021;2297:7-19. doi: 10.1007/978-1-0716-1370-2_2. Methods Mol Biol. 2021. PMID: 33656665
References
-
- Adams R.R., Maiato H., Earnshaw W.C., Carmena M. 2001. Essential roles of Drosophila inner centromere protein (Incenp) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 153:865–880 10.1083/jcb.153.4.865 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

