Kinesin-1 and dynein at the nuclear envelope mediate the bidirectional migrations of nuclei
- PMID: 20921138
- PMCID: PMC2953438
- DOI: 10.1083/jcb.201004118
Kinesin-1 and dynein at the nuclear envelope mediate the bidirectional migrations of nuclei
Abstract
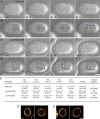
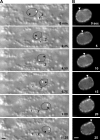
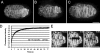
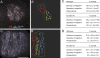
Kinesin-1 and dynein are recruited to the nuclear envelope by the Caenorhabditis elegans klarsicht/ANC-1/Syne homology (KASH) protein UNC-83 to move nuclei. The mechanisms of how these motors are coordinated to mediate nuclear migration are unknown. Time-lapse differential interference contrast and fluorescence imaging of embryonic hypodermal nuclear migration events were used to characterize the kinetics of nuclear migration and determine microtubule dynamics and polarity. Wild-type nuclei display bidirectional movements during migration and are also able to roll past cytoplasmic granules. unc-83, unc-84, and kinesin-1 mutants have severe nuclear migration defects. Without dynein, nuclear migration initiates normally but lacks bidirectional movement and shows defects in nuclear rolling, implicating dynein in resolution of cytoplasmic roadblocks. Microtubules are highly dynamic during nuclear migration. EB1::green fluorescence protein imaging demonstrates that microtubules are polarized in the direction of nuclear migration. This organization of microtubules fits with our model that kinesin-1 moves nuclei forward and dynein functions to move nuclei backward for short stretches to bypass cellular roadblocks.
Figures