Distinct structural rearrangements of the VSV glycoprotein drive membrane fusion
- PMID: 20921141
- PMCID: PMC2953448
- DOI: 10.1083/jcb.201006116
Distinct structural rearrangements of the VSV glycoprotein drive membrane fusion
Abstract
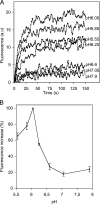
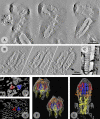
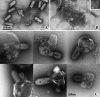
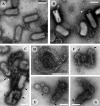
The entry of enveloped viruses into cells requires the fusion of viral and cellular membranes, driven by conformational changes in viral glycoproteins. Many studies have shown that fusion involves the cooperative action of a large number of these glycoproteins, but the underlying mechanisms are unknown. We used electron microscopy and tomography to study the low pH-induced fusion reaction catalyzed by vesicular stomatitis virus glycoprotein (G). Pre- and post-fusion crystal structures were observed on virions at high and low pH, respectively. Individual fusion events with liposomes were also visualized. Fusion appears to be driven by two successive structural rearrangements of G at different sites on the virion. Fusion is initiated at the flat base of the particle. Glycoproteins located outside the contact zone between virions and liposomes then reorganize into regular arrays. We suggest that the formation of these arrays, which have been shown to be an intrinsic property of the G ectodomain, induces membrane constraints, achieving the fusion reaction.
Figures




References
-
- Brown J.C., Newcomb W.W., Lawrenz-Smith S. 1988. pH-dependent accumulation of the vesicular stomatitis virus glycoprotein at the ends of intact virions. Virology. 167:625–629 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

