Effects of the putative transcriptional regulator IclR on Francisella tularensis pathogenesis
- PMID: 20921148
- PMCID: PMC2981306
- DOI: 10.1128/IAI.00544-10
Effects of the putative transcriptional regulator IclR on Francisella tularensis pathogenesis
Abstract
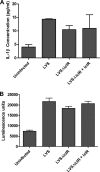
Francisella tularensis is a highly virulent Gram-negative bacterium and is the etiological agent of the disease tularemia. IclR, a presumed transcriptional regulator, is required for full virulence of the animal pathogen, F. tularensis subspecies novicida U112 (53). In this study, we investigated the contribution of IclR to the intracellular growth, virulence, and gene regulation of human pathogenic F. tularensis subspecies. Deletion of iclR from the live vaccine strain (LVS) and SchuS4 strain of F. tularensis subsp. holarctica and F. tularensis subsp. tularensis, respectively, did not affect their abilities to replicate within macrophages or epithelial cells. In contrast to F. tularensis subsp. novicida iclR mutants, LVS and SchuS4 ΔiclR strains were as virulent as their wild-type parental strains in intranasal inoculation mouse models of tularemia. Furthermore, wild-type LVS and LVSΔiclR were equally cytotoxic and induced equivalent levels of interleukin-1β expression by infected bone marrow-derived macrophages. Microarray analysis revealed that the relative expression of a limited number of genes differed significantly between LVS wild-type and ΔiclR strains. Interestingly, many of the identified genes were disrupted in LVS and SchuS4 but not in their corresponding F. tularensis subsp. novicida U112 homologs. Thus, despite the impact of iclR deletion on gene expression, and in contrast to the effects of iclR deletion on F. tularensis subsp. novicida virulence, IclR does not contribute significantly to the virulence or pathogenesis of F. tularensis LVS or SchuS4.
Figures

References
-
- Bosio, C. M., and S. W. Dow. 2005. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 175:6792-6801. - PubMed
-
- Centers for Disease Control and Prevention. 2005. Tularemia transmitted by insect bites—Wyoming, 2001-2003. MMWR Morb. Mortal. Wkly. Rep. 54:170-173. - PubMed
-
- Centers for Disease Control and Prevention. 2009. Tularemia—Missouri, 2000-2007. MMWR Morb. Mortal. Wkly. Rep. 58:744-748. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

