Neuroeffector apparatus in gastrointestinal smooth muscle organs
- PMID: 20921202
- PMCID: PMC3010131
- DOI: 10.1113/jphysiol.2010.196030
Neuroeffector apparatus in gastrointestinal smooth muscle organs
Abstract
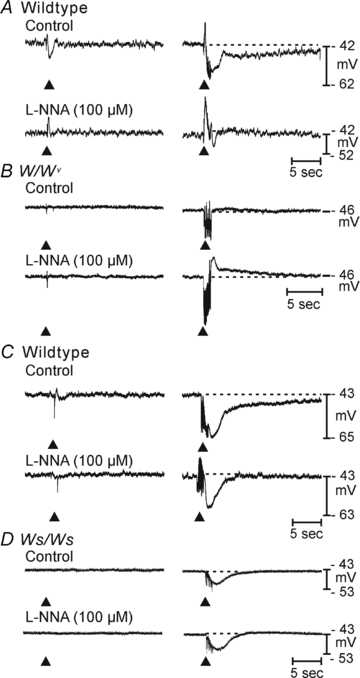
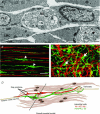
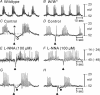
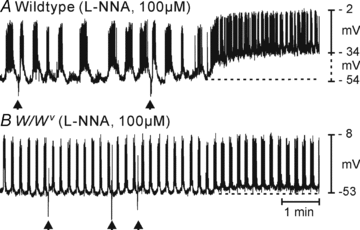
Control of gastrointestinal (GI) movements by enteric motoneurons is critical for orderly processing of food, absorption of nutrients and elimination of wastes. Work over the past several years has suggested that motor neurotransmission is more complicated than simple release of transmitter from nerve terminals and binding of receptors on smooth muscle cells. In fact the 'neuro-effector' junction in the tunica muscularis might consist of synaptic-like connectivity with specialized cells, and contributions from multiple cell types in integrated post-junctional responses. Interstitial cells of Cajal (ICC) were proposed as potential mediators in motor neurotransmission based on reduced post-junctional responses observed in W mutants that have reduced populations of ICC. More recent studies on W mutants have contradicted the original findings, and suggested that ICC may not be significant players in motor neurotransmission. This review examines the evidence for and against the role of ICC in motor neurotransmission and outlines areas for additional investigation that would help further resolve this controversy.
Figures



References
-
- Albertí E, Mikkelsen HB, Wang XY, Díaz M, Larsen JO, Huizinga JD, Jiménez M. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–G1510. - PubMed
-
- Auintas LE, Noël F. Mechanisms of adaptive supersensitivity in vas deferens. Auton Neurosci. 2009;146:38–46. - PubMed
-
- Beckett EA, Takeda Y, Yanase H, Sanders KM, Ward SM. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol. 2005;493:193–206. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

