Colorectal cancer molecular biology moves into clinical practice
- PMID: 20921207
- PMCID: PMC3006043
- DOI: 10.1136/gut.2009.206250
Colorectal cancer molecular biology moves into clinical practice
Abstract
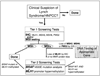
The promise of personalised medicine is now a clinical reality, with colorectal cancer genetics at the forefront of this next major advance in clinical medicine. This is no more evident than in the recent advances in testing of colorectal cancers for specific molecular alterations in order to guide treatment with the monoclonal antibody therapies cetuximab and panitumumab, which target the epidermal growth factor receptor. In this review, genetic mechanisms of colorectal cancer and how these alterations relate to emerging biomarkers for early detection and risk stratification (diagnostic markers), prognosis (prognostic markers) and the prediction of treatment responses (predictive markers) are examined.
Figures


References
-
- European Medicines Agency. Committee for Medicinal Products for Human Use May 2008 Plenary Meeting monthly report. 2008. http://www.emea.europa.eu/pdfs/human/press/pr/27923508en.pdf.
-
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Colon Cancer V.2.2010. 2010. http://www.nccn.org/professionals/physician_gls/PDF/colon.pdf. - PubMed
-
- Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. - PubMed
-
- Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
