Mutation analysis of the presenilin 1 N-terminal domain reveals a broad spectrum of gamma-secretase activity toward amyloid precursor protein and other substrates
- PMID: 20921220
- PMCID: PMC2992238
- DOI: 10.1074/jbc.M110.132613
Mutation analysis of the presenilin 1 N-terminal domain reveals a broad spectrum of gamma-secretase activity toward amyloid precursor protein and other substrates
Abstract
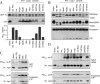
The γ-secretase protein complex executes the intramembrane proteolysis of amyloid precursor protein (APP), which releases Alzheimer disease β-amyloid peptide. In addition to APP, γ-secretase also cleaves several other type I membrane protein substrates including Notch1 and N-cadherin. γ-Secretase is made of four integral transmembrane protein subunits: presenilin (PS), nicastrin, APH1, and PEN2. Multiple lines of evidence indicate that a heteromer of PS-derived N- and C-terminal fragments functions as the catalytic subunit of γ-secretase. Only limited information is available on the domains within each subunit involved in the recognition and recruitment of diverse substrates and the transfer of substrates to the catalytic site. Here, we performed mutagenesis of two domains of PS1, namely the first luminal loop domain (LL1) and the second transmembrane domain (TM2), and analyzed PS1 endoproteolysis as well as the catalytic activities of PS1 toward APP, Notch, and N-cadherin. Our results show that distinct residues within LL1 and TM2 domains as well as the length of the LL1 domain are critical for PS1 endoproteolysis, but not for PS1 complex formation with nicastrin, APH1, and PEN2. Furthermore, our experimental PS1 mutants formed γ-secretase complexes with distinct catalytic properties toward the three substrates examined in this study; however, the mutations did not affect PS1 interaction with the substrates. We conclude that the N-terminal LL1 and TM2 domains are critical for PS1 endoproteolysis and the coordination between the putative substrate-docking site and the catalytic core of the γ-secretase.
Figures

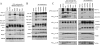



Similar articles
-
Three-amino acid spacing of presenilin endoproteolysis suggests a general stepwise cleavage of gamma-secretase-mediated intramembrane proteolysis.J Neurosci. 2010 Jun 9;30(23):7853-62. doi: 10.1523/JNEUROSCI.1443-10.2010. J Neurosci. 2010. PMID: 20534834 Free PMC article.
-
Gamma-secretase composed of PS1/Pen2/Aph1a can cleave notch and amyloid precursor protein in the absence of nicastrin.J Neurosci. 2010 Feb 3;30(5):1648-56. doi: 10.1523/JNEUROSCI.3826-09.2010. J Neurosci. 2010. PMID: 20130175 Free PMC article.
-
Specific Mutations in Aph1 Cause γ-Secretase Activation.Int J Mol Sci. 2022 Jan 3;23(1):507. doi: 10.3390/ijms23010507. Int J Mol Sci. 2022. PMID: 35008932 Free PMC article.
-
Toward the structure of presenilin/γ-secretase and presenilin homologs.Biochim Biophys Acta. 2013 Dec;1828(12):2886-97. doi: 10.1016/j.bbamem.2013.04.015. Biochim Biophys Acta. 2013. PMID: 24099007 Free PMC article. Review.
-
The Alzheimer's disease-associated gamma-secretase complex: functional domains in the presenilin 1 protein.Physiol Behav. 2007 Sep 10;92(1-2):115-20. doi: 10.1016/j.physbeh.2007.05.037. Epub 2007 May 21. Physiol Behav. 2007. PMID: 17588625 Review.
Cited by
-
Allosteric regulation of γ-secretase activity by a phenylimidazole-type γ-secretase modulator.Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):10544-9. doi: 10.1073/pnas.1402171111. Epub 2014 Jul 9. Proc Natl Acad Sci U S A. 2014. PMID: 25009180 Free PMC article.
-
3,5-bis(2,4-difluorobenzylidene)-4-piperidone, a novel compound that affects pancreatic cancer growth and angiogenesis.Mol Cancer Ther. 2011 Nov;10(11):2146-56. doi: 10.1158/1535-7163.MCT-11-0399. Epub 2011 Sep 2. Mol Cancer Ther. 2011. PMID: 21890747 Free PMC article.
-
Novel GαS-protein signaling associated with membrane-tethered amyloid precursor protein intracellular domain.J Neurosci. 2012 Feb 1;32(5):1714-29. doi: 10.1523/JNEUROSCI.5433-11.2012. J Neurosci. 2012. PMID: 22302812 Free PMC article.
-
Structure and Function of the γ-Secretase Complex.Biochemistry. 2019 Jul 9;58(27):2953-2966. doi: 10.1021/acs.biochem.9b00401. Epub 2019 Jun 25. Biochemistry. 2019. PMID: 31198028 Free PMC article.
-
Polar transmembrane-based amino acids in presenilin 1 are involved in endoplasmic reticulum localization, Pen2 protein binding, and γ-secretase complex stabilization.J Biol Chem. 2011 Nov 4;286(44):38390-38396. doi: 10.1074/jbc.M111.252429. Epub 2011 Sep 13. J Biol Chem. 2011. PMID: 21914807 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

