Deceleration of the E1P-E2P transition and ion transport by mutation of potentially salt bridge-forming residues Lys-791 and Glu-820 in gastric H+/K+-ATPase
- PMID: 20921224
- PMCID: PMC2998106
- DOI: 10.1074/jbc.M110.133470
Deceleration of the E1P-E2P transition and ion transport by mutation of potentially salt bridge-forming residues Lys-791 and Glu-820 in gastric H+/K+-ATPase
Abstract
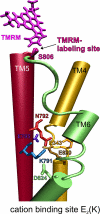
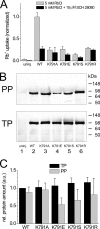
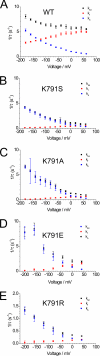
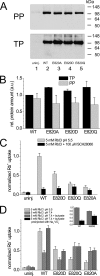
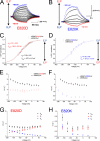
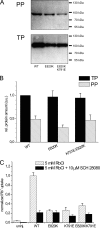
A lysine residue within the highly conserved center of the fifth transmembrane segment in P(IIC)-type ATPase α-subunits is uniquely found in H,K-ATPases instead of a serine in all Na,K-ATPase isoforms. Because previous studies suggested a prominent role of this residue in determining the electrogenicity of non-gastric H,K-ATPase and in pK(a) modulation of the proton-translocating residues in the gastric H,K-ATPases as well, we investigated its functional significance for ion transport by expressing several Lys-791 variants of the gastric H,K-ATPase in Xenopus oocytes. Although the mutant proteins were all detected at the cell surface, none of the investigated mutants displayed any measurable K(+)-induced stationary currents. In Rb(+) uptake measurements, replacement of Lys-791 by Arg, Ala, Ser, and Glu substantially impaired transport activity and reduced the sensitivity toward the E(2)-specific inhibitor SCH28080. Furthermore, voltage clamp fluorometry using a reporter site in the TM5/TM6 loop for labeling with tetra-methylrhodamine-6-maleimide revealed markedly changed fluorescence signals. All four investigated mutants exhibited a strong shift toward the E(1)P state, in agreement with their reduced SCH28080 sensitivity, and an about 5-10-fold decreased forward rate constant of the E(1)P ↔ E(2)P conformational transition, thus explaining the E(1)P shift and the reduced Rb(+) transport activity. When Glu-820 in TM6 adjacent to Lys-791 was replaced by non-charged or positively charged amino acids, severe effects on fluorescence signals and Rb(+) transport were also observed, whereas substitution by aspartate was less disturbing. These results suggest that formation of an E(2)P-stabilizing interhelical salt bridge is essential to prevent futile proton exchange cycles of H(+) pumping P-type ATPases.
Figures


Similar articles
-
E2P state stabilization by the N-terminal tail of the H,K-ATPase beta-subunit is critical for efficient proton pumping under in vivo conditions.J Biol Chem. 2009 Jul 24;284(30):20147-54. doi: 10.1074/jbc.M109.005769. Epub 2009 Jun 2. J Biol Chem. 2009. PMID: 19491099 Free PMC article.
-
Conformational dynamics of Na+/K+- and H+/K+-ATPase probed by voltage clamp fluorometry.Ann N Y Acad Sci. 2003 Apr;986:31-8. doi: 10.1111/j.1749-6632.2003.tb07136.x. Ann N Y Acad Sci. 2003. PMID: 12763772
-
Electrogenicity of Na,K- and H,K-ATPase activity and presence of a positively charged amino acid in the fifth transmembrane segment.J Biol Chem. 2003 May 23;278(21):19237-44. doi: 10.1074/jbc.M300946200. Epub 2003 Mar 12. J Biol Chem. 2003. PMID: 12637496
-
Structural aspects of the gastric H,K ATPase.Ann N Y Acad Sci. 1997 Nov 3;834:65-76. doi: 10.1111/j.1749-6632.1997.tb52226.x. Ann N Y Acad Sci. 1997. PMID: 9405786 Review.
-
[Structure-function study on gastric proton pump].Yakugaku Zasshi. 1997 Jul;117(7):379-93. doi: 10.1248/yakushi1947.117.7_379. Yakugaku Zasshi. 1997. PMID: 9261211 Review. Japanese.
Cited by
-
Cryo-EM structure of gastric H+,K+-ATPase with a single occupied cation-binding site.Proc Natl Acad Sci U S A. 2012 Nov 6;109(45):18401-6. doi: 10.1073/pnas.1212294109. Epub 2012 Oct 22. Proc Natl Acad Sci U S A. 2012. PMID: 23091039 Free PMC article.
-
Control of gastric H,K-ATPase activity by cations, voltage and intracellular pH analyzed by voltage clamp fluorometry in Xenopus oocytes.PLoS One. 2012;7(3):e33645. doi: 10.1371/journal.pone.0033645. Epub 2012 Mar 20. PLoS One. 2012. PMID: 22448261 Free PMC article.
-
Specific protonation of acidic residues confers K+ selectivity to the gastric proton pump.J Biol Chem. 2024 Jan;300(1):105542. doi: 10.1016/j.jbc.2023.105542. Epub 2023 Dec 10. J Biol Chem. 2024. PMID: 38072058 Free PMC article.
-
Displacement of the Na+/K+ pump's transmembrane domains demonstrates conserved conformational changes in P-type 2 ATPases.Proc Natl Acad Sci U S A. 2021 Feb 23;118(8):e2019317118. doi: 10.1073/pnas.2019317118. Proc Natl Acad Sci U S A. 2021. PMID: 33597302 Free PMC article.
-
Gastric proton pump with two occluded K+ engineered with sodium pump-mimetic mutations.Nat Commun. 2021 Sep 29;12(1):5709. doi: 10.1038/s41467-021-26024-1. Nat Commun. 2021. PMID: 34588453 Free PMC article.
References
-
- Geering K. (2001) J. Bioenerg. Biomembr. 33, 425–438 - PubMed
-
- Sachs G., Chang H. H., Rabon E., Schackman R., Lewin M., Saccomani G. (1976) J. Biol. Chem. 251, 7690–7698 - PubMed
-
- Burnay M., Crambert G., Kharoubi-Hess S., Geering K., Horisberger J. D. (2001) Am. J. Physiol. Renal. Physiol. 281, F869–F874 - PubMed
-
- van der Hijden H. T., Grell E., de Pont J. J., Bamberg E. (1990) J. Membr. Biol. 114, 245–256 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

