Deceleration of the E1P-E2P transition and ion transport by mutation of potentially salt bridge-forming residues Lys-791 and Glu-820 in gastric H+/K+-ATPase
- PMID: 20921224
- PMCID: PMC2998106
- DOI: 10.1074/jbc.M110.133470
Deceleration of the E1P-E2P transition and ion transport by mutation of potentially salt bridge-forming residues Lys-791 and Glu-820 in gastric H+/K+-ATPase
Abstract
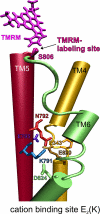
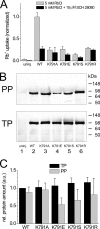
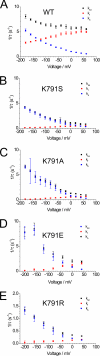
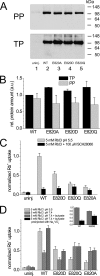
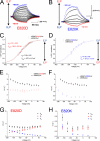
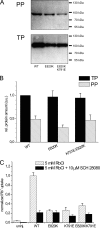
A lysine residue within the highly conserved center of the fifth transmembrane segment in P(IIC)-type ATPase α-subunits is uniquely found in H,K-ATPases instead of a serine in all Na,K-ATPase isoforms. Because previous studies suggested a prominent role of this residue in determining the electrogenicity of non-gastric H,K-ATPase and in pK(a) modulation of the proton-translocating residues in the gastric H,K-ATPases as well, we investigated its functional significance for ion transport by expressing several Lys-791 variants of the gastric H,K-ATPase in Xenopus oocytes. Although the mutant proteins were all detected at the cell surface, none of the investigated mutants displayed any measurable K(+)-induced stationary currents. In Rb(+) uptake measurements, replacement of Lys-791 by Arg, Ala, Ser, and Glu substantially impaired transport activity and reduced the sensitivity toward the E(2)-specific inhibitor SCH28080. Furthermore, voltage clamp fluorometry using a reporter site in the TM5/TM6 loop for labeling with tetra-methylrhodamine-6-maleimide revealed markedly changed fluorescence signals. All four investigated mutants exhibited a strong shift toward the E(1)P state, in agreement with their reduced SCH28080 sensitivity, and an about 5-10-fold decreased forward rate constant of the E(1)P ↔ E(2)P conformational transition, thus explaining the E(1)P shift and the reduced Rb(+) transport activity. When Glu-820 in TM6 adjacent to Lys-791 was replaced by non-charged or positively charged amino acids, severe effects on fluorescence signals and Rb(+) transport were also observed, whereas substitution by aspartate was less disturbing. These results suggest that formation of an E(2)P-stabilizing interhelical salt bridge is essential to prevent futile proton exchange cycles of H(+) pumping P-type ATPases.
Figures


References
-
- Geering K. (2001) J. Bioenerg. Biomembr. 33, 425–438 - PubMed
-
- Sachs G., Chang H. H., Rabon E., Schackman R., Lewin M., Saccomani G. (1976) J. Biol. Chem. 251, 7690–7698 - PubMed
-
- Burnay M., Crambert G., Kharoubi-Hess S., Geering K., Horisberger J. D. (2001) Am. J. Physiol. Renal. Physiol. 281, F869–F874 - PubMed
-
- van der Hijden H. T., Grell E., de Pont J. J., Bamberg E. (1990) J. Membr. Biol. 114, 245–256 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

