Three- and four-repeat Tau coassemble into heterogeneous filaments: an implication for Alzheimer disease
- PMID: 20921227
- PMCID: PMC2988394
- DOI: 10.1074/jbc.M110.185728
Three- and four-repeat Tau coassemble into heterogeneous filaments: an implication for Alzheimer disease
Abstract
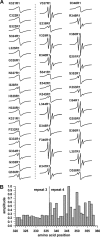
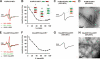
Tau filaments are the pathological hallmark of numerous neurodegenerative diseases including Alzheimer disease, Pick disease, and progressive supranuclear palsy. In the adult human brain, six isoforms are expressed that differ by the presence or absence of the second of four semiconserved repeats. As a consequence, half of the tau isoforms have three repeats (3R tau), whereas the other half of the isoforms have four repeats (4R tau). Tauopathies can be characterized based on the isoform composition of their filaments. Alzheimer disease filamentous inclusions contain all isoforms. Pick disease filaments contain 3R tau. Progressive supranuclear palsy filaments contain 4R tau. Here, we used site-directed spin labeling of recombinant tau in conjunction with electron paramagnetic resonance spectroscopy to obtain structural insights into these filaments. We find that filaments of 4R tau and 3R tau share a highly ordered core structure in the third repeat with parallel, in-register arrangement of β-strands. This structure is conserved regardless of whether full-length isoforms (htau40 and htau23) or truncated constructs (K18 and K19) are used. When mixed, 3R tau and 4R tau coassemble into heterogeneous filaments. These filaments share the highly ordered core in the third repeat; however, they differ in their overall composition. Our findings indicate that at least three distinct types of filaments exist: homogeneous 3R tau, homogeneous 4R tau, and heterogeneous 3R/4R tau. These results suggest that individual filaments found in Alzheimer disease are structurally distinct from those in the 3R and 4R tauopathies.
Figures



Similar articles
-
Structural disorder in four-repeat Tau fibrils reveals a new mechanism for barriers to cross-seeding of Tau isoforms.J Biol Chem. 2018 Nov 9;293(45):17336-17348. doi: 10.1074/jbc.RA118.005316. Epub 2018 Sep 21. J Biol Chem. 2018. PMID: 30242125 Free PMC article.
-
Structures of filaments from Pick's disease reveal a novel tau protein fold.Nature. 2018 Sep;561(7721):137-140. doi: 10.1038/s41586-018-0454-y. Epub 2018 Aug 29. Nature. 2018. PMID: 30158706 Free PMC article.
-
Molecular Simulations Reveal Distinct Energetic and Kinetic Binding Properties of [18F]PI-2620 on Tau Filaments from 3R/4R and 4R Tauopathies.ACS Chem Neurosci. 2022 Jul 20;13(14):2222-2234. doi: 10.1021/acschemneuro.2c00291. Epub 2022 Jun 28. ACS Chem Neurosci. 2022. PMID: 35762647
-
Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick's disease.Brain Pathol. 1999 Oct;9(4):681-93. doi: 10.1111/j.1750-3639.1999.tb00550.x. Brain Pathol. 1999. PMID: 10517507 Free PMC article. Review.
-
[Neuropathology of tauopathy].Brain Nerve. 2013 Dec;65(12):1445-58. Brain Nerve. 2013. PMID: 24323931 Review. Japanese.
Cited by
-
Revealing Conformational Variants of Solution-Phase Intrinsically Disordered Tau Protein at the Single-Molecule Level.Angew Chem Int Ed Engl. 2017 Dec 4;56(49):15584-15588. doi: 10.1002/anie.201708242. Epub 2017 Nov 14. Angew Chem Int Ed Engl. 2017. PMID: 29063723 Free PMC article.
-
Cellular Uptake of Tau Aggregates Triggers Disulfide Bond Formation in Four-Repeat Tau Monomers.ACS Chem Neurosci. 2025 Jan 15;16(2):171-180. doi: 10.1021/acschemneuro.4c00607. Epub 2024 Dec 23. ACS Chem Neurosci. 2025. PMID: 39714208 Free PMC article.
-
Potential mechanisms and implications for the formation of tau oligomeric strains.Crit Rev Biochem Mol Biol. 2016 Nov/Dec;51(6):482-496. doi: 10.1080/10409238.2016.1226251. Epub 2016 Sep 21. Crit Rev Biochem Mol Biol. 2016. PMID: 27650389 Free PMC article. Review.
-
Tau Protein and Its Role in Blood-Brain Barrier Dysfunction.Front Mol Neurosci. 2020 Sep 30;13:570045. doi: 10.3389/fnmol.2020.570045. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33100967 Free PMC article. Review.
-
Prion and Prion-Like Protein Strains: Deciphering the Molecular Basis of Heterogeneity in Neurodegeneration.Viruses. 2019 Mar 14;11(3):261. doi: 10.3390/v11030261. Viruses. 2019. PMID: 30875755 Free PMC article. Review.
References
-
- Goedert M., Spillantini M. G. (2006) Science 314, 777–781 - PubMed
-
- Lee V. M., Goedert M., Trojanowski J. Q. (2001) Annu. Rev. Neurosci. 24, 1121–1159 - PubMed
-
- Hutton M., Lendon C. L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., Hackett J., Adamson J., Lincoln S., Dickson D., Davies P., Petersen R. C., Stevens M., de Graaff E., Wauters E., van Baren J., Hillebrand M., Joosse M., Kwon J. M., Nowotny P., Che L. K., Norton J., Morris J. C., Reed L. A., Trojanowski J., Basun H., Lannfelt L., Neystat M., Fahn S., Dark F., Tannenberg T., Dodd P. R., Hayward N., Kwok J. B., Schofield P. R., Andreadis A., Snowden J., Craufurd D., Neary D., Owen F., Oostra B. A., Hardy J., Goate A., van Swieten J., Mann D., Lynch T., Heutink P. (1998) Nature 393, 702–705 - PubMed
-
- Poorkaj P., Bird T. D., Wijsman E., Nemens E., Garruto R. M., Anderson L., Andreadis A., Wiederholt W. C., Raskind M., Schellenberg G. D. (1998) Ann. Neurol. 43, 815–825 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

