Direct and specific activation of human inward rectifier K+ channels by membrane phosphatidylinositol 4,5-bisphosphate
- PMID: 20921230
- PMCID: PMC2988318
- DOI: 10.1074/jbc.C110.186692
Direct and specific activation of human inward rectifier K+ channels by membrane phosphatidylinositol 4,5-bisphosphate
Abstract
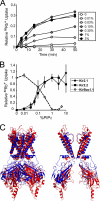
Many ion channels are modulated by phosphatidylinositol 4,5-bisphosphate (PIP(2)), but studies examining the PIP(2) dependence of channel activity have been limited to cell expression systems, which present difficulties for controlling membrane composition. We have characterized the PIP(2) dependence of purified human Kir2.1 and Kir2.2 activity using (86)Rb(+) flux and patch clamp assays in liposomes of defined composition. We definitively show that these channels are directly activated by PIP(2) and that PIP(2) is absolutely required in the membrane for channel activity. The results provide the first quantitative description of the dependence of eukaryotic Kir channel function on PIP(2) levels in the membrane; Kir2.1 shows measureable activity in as little as 0.01% PIP(2), and open probability increases to ∼0.4 at 1% PIP(2). Activation of Kir2.1 by phosphatidylinositol phosphates is also highly selective for PIP(2); PI, PI(4)P, and PI(5)P do not activate channels, and PI(3,4,5)P(3) causes minimal activity. The PIP(2) dependence of eukaryotic Kir activity is almost exactly opposite that of KirBac1.1, which shows marked inhibition by PIP(2). This raises the interesting hypothesis that PIP(2) activation of eukaryotic channels reflects an evolutionary adaptation of the channel to the appearance of PIP(2) in the eukaryotic cell membrane.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

