A requirement for FcγR in antibody-mediated bacterial toxin neutralization
- PMID: 20921285
- PMCID: PMC2964574
- DOI: 10.1084/jem.20100995
A requirement for FcγR in antibody-mediated bacterial toxin neutralization
Abstract
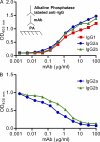
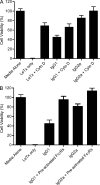
One important function of humoral immunity is toxin neutralization. The current view posits that neutralization results from antibody-mediated interference with the binding of toxins to their targets, a phenomenon viewed as dependent only on antibody specificity. To investigate the role of antibody constant region function in toxin neutralization, we generated IgG2a and IgG2b variants of the Bacillus anthracis protective antigen-binding IgG1 monoclonal antibody (mAb) 19D9. These antibodies express identical variable regions and display the same specificity. The efficacy of antibody-mediated neutralization was IgG2a > IgG2b > IgG1, and neutralization activity required competent Fcγ receptor (FcγR). The IgG2a mAb prevented lethal toxin cell killing and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase cleavage more efficiently than the IgG1 mAb. Passive immunization with IgG1 and IgG2a mAb protected wild-type mice, but not FcγR-deficient mice, against B. anthracis infection. These results establish that constant region isotype influences toxin neutralization efficacy of certain antibodies through a mechanism that requires engagement of FcγR. These findings highlight a new parameter for evaluating vaccine responses and the possibility of harnessing optimal FcγR interactions in the design of passive immunization strategies.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI033142/AI/NIAID NIH HHS/United States
- A1033774/PHS HHS/United States
- R01 HL059842/HL/NHLBI NIH HHS/United States
- 5U54AI05715807/AI/NIAID NIH HHS/United States
- T32 AI007501/AI/NIAID NIH HHS/United States
- 2R01 CA102705/CA/NCI NIH HHS/United States
- U54 AI57158/AI/NIAID NIH HHS/United States
- AI033142/AI/NIAID NIH HHS/United States
- 2R01CA72649/CA/NCI NIH HHS/United States
- HL059842-3/HL/NHLBI NIH HHS/United States
- U54 AI057158/AI/NIAID NIH HHS/United States
- R01 CA102705/CA/NCI NIH HHS/United States
- A1052733/PHS HHS/United States
- R37 AI033142/AI/NIAID NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
- R01 CA072649/CA/NCI NIH HHS/United States
- T32-AI007501/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

