A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma
- PMID: 20921287
- PMCID: PMC2964570
- DOI: 10.1084/jem.20101376
A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma
Abstract
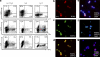
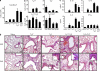
The inflammatory cytokine interleukin (IL)-17 is involved in the pathogenesis of allergic diseases. However, the identity and functions of IL-17-producing T cells during the pathogenesis of allergic diseases remain unclear. Here, we report a novel subset of T(H)2 memory/effector cells that coexpress the transcription factors GATA3 and RORγt and coproduce T(H)17 and T(H)2 cytokines. Classical T(H)2 memory/effector cells had the potential to produce IL-17 after stimulation with proinflammatory cytokines IL-1β, IL-6, and IL-21. The number of IL-17-T(H)2 cells was significantly increased in blood of patients with atopic asthma. In a mouse model of allergic lung diseases, IL-17-producing CD4(+) T(H)2 cells were induced in the inflamed lung and persisted as the dominant IL-17-producing T cell population during the chronic stage of asthma. Treating cultured bronchial epithelial cells with IL-17 plus T(H)2 cytokines induced strong up-regulation of chemokine eotaxin-3, Il8, Mip1b, and Groa gene expression. Compared with classical T(H)17 and T(H)2 cells, antigen-specific IL-17-producing T(H)2 cells induced a profound influx of heterogeneous inflammatory leukocytes and exacerbated asthma. Our findings highlight the plasticity of T(H)2 memory cells and suggest that IL-17-producing T(H)2 cells may represent the key pathogenic T(H)2 cells promoting the exacerbation of allergic asthma.
Figures
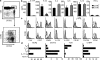





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

