The effect of sevelamer carbonate and lanthanum carbonate on the pharmacokinetics of oral calcitriol
- PMID: 20921291
- PMCID: PMC3084439
- DOI: 10.1093/ndt/gfq598
The effect of sevelamer carbonate and lanthanum carbonate on the pharmacokinetics of oral calcitriol
Abstract
Background: Lanthanum carbonate and sevelamer carbonate are non-calcium-based phosphate binders used to manage hyperphosphataemia in patients with chronic kidney disease (CKD). Patients with CKD may require intravenous or oral active vitamin D. We investigated the effects of lanthanum carbonate and sevelamer carbonate on the bioavailability of oral calcitriol.
Methods: This was a three-period, crossover study in healthy volunteers. Forty-one individuals were randomized to one of six possible sequences, each consisting of three treatment periods separated by washouts. The treatments were calcitriol (1 μg at lunch), calcitriol with lanthanum carbonate (3000 mg/day) and calcitriol with sevelamer carbonate (7200 mg/day). Serum calcitriol levels were assessed at baseline and throughout the study.
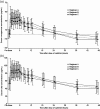
Results: Co-administration of lanthanum carbonate with calcitriol had no significant effect on area under the curve over 48 h (AUC(0-48)) for serum exogenous calcitriol [least-squares (LS) mean, calcitriol with lanthanum carbonate vs calcitriol alone: 429 pg h/mL vs 318 pg h/mL, respectively; P = 0.171]. Similarly, there was no significant effect on maximum concentration (C(max)). In contrast, co-administration with sevelamer was associated with a significant reduction in bioavailability parameters for calcitriol (calcitriol with sevelamer carbonate vs calcitriol alone, LS mean AUC(0-48): 137 pg h/mL vs 318 pg h/mL, respectively; P = 0.024; LS mean C(max): 40.1 pg/mL vs 49.7 pg/mL, respectively; P < 0.001).
Conclusions: Sevelamer carbonate significantly reduces serum concentrations of exogenous calcitriol when administered concomitantly with oral calcitriol, whereas lanthanum carbonate has no significant effect. This should be considered when treating CKD patients who require phosphate binders and oral vitamin D.
Figures

Similar articles
-
Differences in gastrointestinal calcium absorption after the ingestion of calcium-free phosphate binders.Am J Physiol Renal Physiol. 2014 Jan 1;306(1):F61-7. doi: 10.1152/ajprenal.00219.2013. Epub 2013 Nov 6. Am J Physiol Renal Physiol. 2014. PMID: 24197066 Clinical Trial.
-
Comparison of dietary phosphate absorption after single doses of lanthanum carbonate and sevelamer carbonate in healthy volunteers: a balance study.Am J Kidney Dis. 2011 May;57(5):700-6. doi: 10.1053/j.ajkd.2010.11.028. Epub 2011 Feb 26. Am J Kidney Dis. 2011. PMID: 21354682 Clinical Trial.
-
A comparative study of phosphate binders in patients with end stage kidney disease undergoing hemodialysis.Saudi J Kidney Dis Transpl. 2014 May;25(3):530-8. doi: 10.4103/1319-2442.132167. Saudi J Kidney Dis Transpl. 2014. PMID: 24821148
-
A comparative review of the efficacy and safety of established phosphate binders: calcium, sevelamer, and lanthanum carbonate.Curr Med Res Opin. 2007 Dec;23(12):3167-75. doi: 10.1185/030079907X242719. Curr Med Res Opin. 2007. PMID: 17991307 Review.
-
Sevelamer carbonate.Ann Pharmacother. 2010 Jan;44(1):127-34. doi: 10.1345/aph.1M291. Epub 2009 Dec 2. Ann Pharmacother. 2010. PMID: 19955298 Review.
Cited by
-
Pharmacology, efficacy and safety of oral phosphate binders.Nat Rev Nephrol. 2011 Sep 6;7(10):578-89. doi: 10.1038/nrneph.2011.112. Nat Rev Nephrol. 2011. PMID: 21894188 Review.
-
Exploring co-dispensed drug use in patients on sevelamer or polystyrene sulfonate to identify potential novel binding interactions: a cross sectional in silico study : Potential novel binding interactions with resins.Int J Clin Pharm. 2022 Apr;44(2):389-398. doi: 10.1007/s11096-021-01355-7. Epub 2021 Nov 30. Int J Clin Pharm. 2022. PMID: 34850339
-
Phosphate Binders and Nonphosphate Effects in the Gastrointestinal Tract.J Ren Nutr. 2020 Jan;30(1):4-10. doi: 10.1053/j.jrn.2019.01.004. Epub 2019 Mar 4. J Ren Nutr. 2020. PMID: 30846238 Free PMC article. Review.
-
Calcitriol-mediated hypercalcemia secondary to granulomatous disease caused by soft-tissue filler injection: a case report.Clin Cases Miner Bone Metab. 2017 Sep-Dec;14(3):340-346. doi: 10.11138/ccmbm/2017.14.3.340. Epub 2017 Dec 27. Clin Cases Miner Bone Metab. 2017. PMID: 29354165 Free PMC article.
-
Calcitriol, calcidiol, parathyroid hormone, and fibroblast growth factor-23 interactions in chronic kidney disease.J Vet Emerg Crit Care (San Antonio). 2013 Mar-Apr;23(2):134-62. doi: 10.1111/vec.12036. J Vet Emerg Crit Care (San Antonio). 2013. PMID: 23566108 Free PMC article. Review.
References
-
- LaClair RE, Hellman RN, Karp SL, et al. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026–1033. - PubMed
-
- Pesenson A, Maski M, Kaufman J. Prevalence of 25-OH vitamin D deficiency in patients with chronic kidney disease and effects of correction following KDOQI guidelines. Poster Presentation at the American Society of Nephrology Renal Week; San Diego, CA, USA. 2006. pp. 14–19.
-
- Craver L, Marco MP, Martinez I, et al. Mineral metabolism parameters throughout chronic kidney disease stages 1–5—achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22:1171–1176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

