Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy
- PMID: 20921363
- PMCID: PMC2964221
- DOI: 10.1073/pnas.1011368107
Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy
Abstract
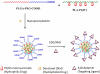
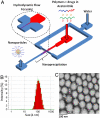
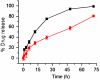
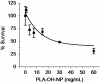
The genomic revolution has identified therapeutic targets for a plethora of diseases, creating a need to develop robust technologies for combination drug therapy. In the present work, we describe a self-assembled polymeric nanoparticle (NP) platform to target and control precisely the codelivery of drugs with varying physicochemical properties to cancer cells. As proof of concept, we codelivered cisplatin and docetaxel (Dtxl) to prostate cancer cells with synergistic cytotoxicity. A polylactide (PLA) derivative with pendant hydroxyl groups was prepared and conjugated to a platinum(IV) [Pt(IV)] prodrug, c,t,c-[Pt(NH(3))(2)(O(2)CCH(2)CH(2)COOH)(OH)Cl(2)] [PLA-Pt(IV)]. A blend of PLA-Pt(IV) functionalized polymer and carboxyl-terminated poly(D,L-lactic-co-glycolic acid)-block-poly(ethylene glycol) copolymer in the presence or absence of Dtxl, was converted, in microfluidic channels, to NPs with a diameter of ∼100 nm. This process resulted in excellent encapsulation efficiency (EE) and high loading of both hydrophilic platinum prodrug and hydrophobic Dtxl with reproducible EEs and loadings. The surface of the NPs was derivatized with the A10 aptamer, which binds to the prostate-specific membrane antigen (PSMA) on prostate cancer cells. These NPs undergo controlled release of both drugs over a period of 48-72 h. Targeted NPs were internalized by the PSMA-expressing LNCaP cells via endocytosis, and formation of cisplatin 1,2-d(GpG) intrastrand cross-links on nuclear DNA was verified. In vitro toxicities demonstrated superiority of the targeted dual-drug combination NPs over NPs with single drug or nontargeted NPs. This work reveals the potential of a single, programmable nanoparticle to blend and deliver a combination of drugs for cancer treatment.
Conflict of interest statement
Conflict of interest statement: O.C.F. discloses his financial interest in BIND Biosciences and Selecta Biosciences, two biotechnology companies developing nanoparticle technologies for medical applications. BIND and Selecta did not support the aforementioned research, and currently these companies have no rights to any technology or intellectual property developed as part of this research. BIND and Selecta are biopharmaceutical companies founded by O.C.F. and R.L., where they both serve as members of the Board of Directors and Scientific Advisory Board.
Figures


References
-
- Welch DR. Biologic considerations for drug targeting in cancer patients. Cancer Treat Rev. 1987;14:351–358. - PubMed
-
- Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. - PubMed
-
- Jia J, et al. Mechanisms of drug combinations: Interaction and network perspectives. Nat Rev Drug Discovery. 2009;8:111–128. - PubMed
-
- Orjuela P, Gonzalez I, Osorio L. Combination therapy as a strategy to prevent antimalarial drug resistance. Biomedica. 2004;24:423–437. - PubMed
-
- de Gaetano Donati K, Rabagliati R, Iacoviello L, Cauda R. HIV infection, HAART, and endothelial adhesion molecules: Current perspectives. Lancet Infect Dis. 2004;4:213–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

