Controlling the growth and shape of chiral supramolecular polymers in water
- PMID: 20921365
- PMCID: PMC2964246
- DOI: 10.1073/pnas.1009592107
Controlling the growth and shape of chiral supramolecular polymers in water
Abstract
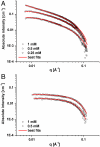
A challenging target in the noncovalent synthesis of nanostructured functional materials is the formation of uniform features that exhibit well-defined properties, e.g., precise control over the aggregate shape, size, and stability. In particular, for aqueous-based one-dimensional supramolecular polymers, this is a daunting task. Here we disclose a strategy based on self-assembling discotic amphiphiles that leads to the control over stack length and shape of ordered, chiral columnar aggregates. By balancing out attractive noncovalent forces within the hydrophobic core of the polymerizing building blocks with electrostatic repulsive interactions on the hydrophilic rim we managed to switch from elongated, rod-like assemblies to small and discrete objects. Intriguingly this rod-to-sphere transition is expressed in a loss of cooperativity in the temperature-dependent self-assembly mechanism. The aggregates were characterized using circular dichroism, UV and 1H-NMR spectroscopy, small angle X-ray scattering, and cryotransmission electron microscopy. In analogy to many systems found in biology, mechanistic details of the self-assembly pathways emphasize the importance of cooperativity as a key feature that dictates the physical properties of the produced supramolecular polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





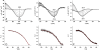
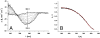

Similar articles
-
Controlling the size, shape and stability of supramolecular polymers in water.J Vis Exp. 2012 Aug 2;(66):e3975. doi: 10.3791/3975. J Vis Exp. 2012. PMID: 22895608 Free PMC article.
-
Controlled supramolecular oligomerization of C3-symmetrical molecules in water: the impact of hydrophobic shielding.Chemistry. 2011 Apr 26;17(18):5193-203. doi: 10.1002/chem.201002976. Epub 2011 Mar 22. Chemistry. 2011. PMID: 21432920
-
Effects of the Mixing Protocol on the Self-Assembling Process of Water Soluble Porphyrins.Int J Mol Sci. 2021 Jan 14;22(2):797. doi: 10.3390/ijms22020797. Int J Mol Sci. 2021. PMID: 33466834 Free PMC article.
-
Molecular Recognition Driven Bioinspired Directional Supramolecular Assembly of Amphiphilic (Macro)molecules and Proteins.Acc Chem Res. 2021 Jun 1;54(11):2670-2682. doi: 10.1021/acs.accounts.1c00195. Epub 2021 May 20. Acc Chem Res. 2021. PMID: 34014638 Review.
-
Secondary Structure in Nonpeptidic Supramolecular Block Copolymers.Acc Chem Res. 2021 May 18;54(10):2397-2408. doi: 10.1021/acs.accounts.1c00028. Epub 2021 Apr 29. Acc Chem Res. 2021. PMID: 33914498 Review.
Cited by
-
Solid-Phase Synthesis of Self-Assembling Multivalent π-Conjugated Peptides.ACS Omega. 2017 Feb 7;2(2):409-419. doi: 10.1021/acsomega.6b00414. eCollection 2017 Feb 28. ACS Omega. 2017. PMID: 31457447 Free PMC article.
-
Positional Order in the Columnar Phase of Lyotropic Chromonic Liquid Crystals Mediated by Ionic Additives.ACS Omega. 2020 Apr 24;5(17):9937-9943. doi: 10.1021/acsomega.0c00229. eCollection 2020 May 5. ACS Omega. 2020. PMID: 32391481 Free PMC article.
-
Thermodynamic insights into the entropically driven self-assembly of amphiphilic dyes in water.Chem Sci. 2019 Aug 20;10(40):9358-9366. doi: 10.1039/c9sc03103k. eCollection 2019 Oct 28. Chem Sci. 2019. PMID: 32110300 Free PMC article.
-
A Kinetic Investigation of the Supramolecular Chiral Self-Assembling Process of Cationic Organometallic (2,2':6',2″-terpyridine)methylplatinum(II) Complexes with Poly(L-glutamic Acid).Int J Mol Sci. 2024 Jan 18;25(2):1176. doi: 10.3390/ijms25021176. Int J Mol Sci. 2024. PMID: 38256248 Free PMC article.
-
Depolymerization of supramolecular polymers by a covalent reaction; transforming an intercalator into a sequestrator.Chem Sci. 2021 Sep 29;12(40):13572-13579. doi: 10.1039/d1sc04545h. eCollection 2021 Oct 20. Chem Sci. 2021. PMID: 34777777 Free PMC article.
References
-
- Whitesides GM, Mathias JP. Molecular self-assembly and nanochemistry. Science. 1991;254:1312–1319. - PubMed
-
- Lehn J-M. Supramolecular Chemistry. Weinheim, Germany: Wiley-VCH; 1995.
-
- Lehn J-M. Toward self-organization and complex matter. Science. 2002;295:2400–2403. - PubMed
-
- Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–2421. - PubMed
-
- Förster S, Antonietti M. Amphiphilic block copolymers in structure-controlled nanomaterial hybrids. Adv Mater. 1998;10:195–217.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

