Direct and enantioselective {alpha}-allylation of ketones via singly occupied molecular orbital (SOMO) catalysis
- PMID: 20921367
- PMCID: PMC2996445
- DOI: 10.1073/pnas.1002845107
Direct and enantioselective {alpha}-allylation of ketones via singly occupied molecular orbital (SOMO) catalysis
Abstract
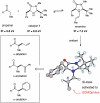
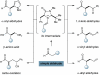
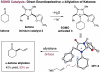
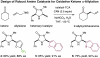
The first enantioselective organocatalytic α-allylation of cyclic ketones has been accomplished via singly occupied molecular orbital catalysis. Geometrically constrained radical cations, forged from the one-electron oxidation of transiently generated enamines, readily undergo allylic alkylation with a variety of commercially available allyl silanes. A reasonable latitude in both the ketone and allyl silane components is readily accommodated in this new transformation. Moreover, three new oxidatively stable imidazolidinone catalysts have been developed that allow cyclic ketones to successfully participate in this transformation. The new catalyst platform has also been exploited in the first catalytic enantioselective α-enolation and α-carbooxidation of ketones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
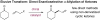

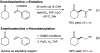
Similar articles
-
Enantioselective organocatalytic singly occupied molecular orbital activation: the enantioselective alpha-enolation of aldehydes.J Am Chem Soc. 2007 Jun 6;129(22):7004-5. doi: 10.1021/ja0719428. Epub 2007 May 12. J Am Chem Soc. 2007. PMID: 17497866 No abstract available.
-
The Intramolecular Asymmetric Allylation of Aldehydes via Organo-SOMO Catalysis: A Novel Approach to Ring Construction.Chem Sci. 2011 Aug 1;2(8):1470-1473. doi: 10.1039/C1SC00176K. Epub 2011 May 19. Chem Sci. 2011. PMID: 23087809 Free PMC article.
-
Enantioselective polyene cyclization via organo-SOMO catalysis.J Am Chem Soc. 2010 Apr 14;132(14):5027-9. doi: 10.1021/ja100185p. J Am Chem Soc. 2010. PMID: 20334384 Free PMC article.
-
Metal-catalyzed silylation of sp3C-H bonds.Chem Soc Rev. 2021 Apr 26;50(8):5062-5085. doi: 10.1039/d0cs01392g. Chem Soc Rev. 2021. PMID: 33629997 Review.
-
Practical Enantioselective Reduction of Ketones Using Oxazaborolidine Catalysts Generated In Situ from Chiral Lactam Alcohols.Molecules. 2018 Sep 20;23(10):2408. doi: 10.3390/molecules23102408. Molecules. 2018. PMID: 30241305 Free PMC article. Review.
Cited by
-
Enantioselective intramolecular aldehyde α-alkylation with simple olefins: direct access to homo-ene products.J Am Chem Soc. 2013 Jun 26;135(25):9358-61. doi: 10.1021/ja4047312. Epub 2013 Jun 12. J Am Chem Soc. 2013. PMID: 23745728 Free PMC article.
-
Nickel-catalyzed switchable asymmetric electrochemical functionalization of alkenes.Sci Adv. 2022 Nov 11;8(45):eadd7134. doi: 10.1126/sciadv.add7134. Epub 2022 Nov 9. Sci Adv. 2022. PMID: 36351023 Free PMC article.
-
Radical Termination via β-Scission Enables Photoenzymatic Allylic Alkylation Using "Ene"-Reductases.ACS Catal. 2022 Aug 5;12(15):9801-9805. doi: 10.1021/acscatal.2c02294. Epub 2022 Jul 27. ACS Catal. 2022. PMID: 37859751 Free PMC article.
-
Enantioselective total synthesis of (-)-minovincine in nine chemical steps: an approach to ketone activation in cascade catalysis.Angew Chem Int Ed Engl. 2013 Oct 18;52(43):11269-72. doi: 10.1002/anie.201305171. Epub 2013 Sep 2. Angew Chem Int Ed Engl. 2013. PMID: 24000234 Free PMC article.
-
Organocatalysis.Proc Natl Acad Sci U S A. 2010 Nov 30;107(48):20618-9. doi: 10.1073/pnas.1016087107. Proc Natl Acad Sci U S A. 2010. PMID: 21119011 Free PMC article.
References
-
- Carey FA, Sundberg RJ. Advanced Organic Chemistry Part B: Reactions and Synthesis. 4th Ed. New York: Kluwer Academic/Plenum; 2001. Alkylation of nucleophilic carbon intermediates; pp. 1–47.
-
- Lu Z, Ma S. Metal-catalyzed enantioselective allylation in asymmetric synthesis. Angew Chem Int Edit. 2008;47:258–297. - PubMed
-
- Trost BM, Crawley ML. Asymmetric transition-metal-catalyzed allylic alkylations: Applications in total synthesis. Chem Rev. 2003;103:2921–2944. - PubMed
-
- Braun M, Meier T. New developments in stereoselective palladium-catalyzed allylic alkylations of preformed enolates. Synlett. 2006:661–676.
-
- Doyle AG, Jacobsen EN. Enantioselective alkylations of tributyltin enolates catalyzed by Cr(salen)Cl: Access to enantiomerically enriched all-carbon quaternary centers. J Am Chem Soc. 2005;127:62–63. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

