Structure of bacterial cellulose synthase subunit D octamer with four inner passageways
- PMID: 20921370
- PMCID: PMC2964256
- DOI: 10.1073/pnas.1000601107
Structure of bacterial cellulose synthase subunit D octamer with four inner passageways
Abstract
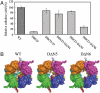
The cellulose synthesizing terminal complex consisting of subunits A, B, C, and D in Acetobacter xylinum spans the outer and inner cell membranes to synthesize and extrude glucan chains, which are assembled into subelementary fibrils and further into a ribbon. We determined the structures of subunit D (AxCeSD/AxBcsD) with both N- and C-terminal His(6) tags, and in complex with cellopentaose. The structure of AxCeSD shows an exquisite cylinder shape (height: ∼65 Å, outer diameter: ∼90 Å, and inner diameter: ∼25 Å) with a right-hand twisted dimer interface on the cylinder wall, formed by octamer as a functional unit. All N termini of the octamer are positioned inside the AxCeSD cylinder and create four passageways. The location of cellopentaoses in the complex structure suggests that four glucan chains are extruded individually through their own passageway along the dimer interface in a twisted manner. The complex structure also shows that the N-terminal loop, especially residue Lys6, seems to be important for cellulose production, as confirmed by in vivo assay using mutant cells with axcesD gene disruption and N-terminus truncation. Taking all results together, a model of the bacterial terminal complex is discussed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
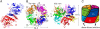

Similar articles
-
Cellulose complementing factor (Ccp) is a new member of the cellulose synthase complex (terminal complex) in Acetobacter xylinum.J Biosci Bioeng. 2013 Jun;115(6):607-12. doi: 10.1016/j.jbiosc.2012.12.021. Epub 2013 Jan 18. J Biosci Bioeng. 2013. PMID: 23333642
-
Cloning and sequencing of the cellulose synthase catalytic subunit gene of Acetobacter xylinum.Plant Mol Biol. 1990 Nov;15(5):673-83. doi: 10.1007/BF00016118. Plant Mol Biol. 1990. PMID: 2151718
-
Extraction of cellulose-synthesizing activity of Gluconacetobacter xylinus by alkylmaltoside.Carbohydr Res. 2011 Dec 13;346(17):2760-8. doi: 10.1016/j.carres.2011.09.031. Epub 2011 Oct 4. Carbohydr Res. 2011. PMID: 22070831
-
The c-di-GMP recognition mechanism of the PilZ domain of bacterial cellulose synthase subunit A.Biochem Biophys Res Commun. 2013 Feb 22;431(4):802-7. doi: 10.1016/j.bbrc.2012.12.103. Epub 2013 Jan 4. Biochem Biophys Res Commun. 2013. PMID: 23291177
-
Purification, crystallization and preliminary X-ray studies of AxCesD required for efficient cellulose biosynthesis in Acetobacter xylinum.Protein Pept Lett. 2008;15(1):115-7. doi: 10.2174/092986608783330422. Protein Pept Lett. 2008. PMID: 18221022
Cited by
-
Crystallographic snapshot of cellulose synthesis and membrane translocation.Nature. 2013 Jan 10;493(7431):181-6. doi: 10.1038/nature11744. Epub 2012 Dec 9. Nature. 2013. PMID: 23222542 Free PMC article.
-
A systematic pipeline for classifying bacterial operons reveals the evolutionary landscape of biofilm machineries.PLoS Comput Biol. 2020 Apr 1;16(4):e1007721. doi: 10.1371/journal.pcbi.1007721. eCollection 2020 Apr. PLoS Comput Biol. 2020. PMID: 32236097 Free PMC article.
-
Glycobiology: Cellulose squeezes through.Nat Chem Biol. 2010 Dec;6(12):883-4. doi: 10.1038/nchembio.480. Nat Chem Biol. 2010. PMID: 21079599 No abstract available.
-
Identification and characterization of non-cellulose-producing mutants of Gluconacetobacter hansenii generated by Tn5 transposon mutagenesis.J Bacteriol. 2013 Nov;195(22):5072-83. doi: 10.1128/JB.00767-13. Epub 2013 Sep 6. J Bacteriol. 2013. PMID: 24013627 Free PMC article.
-
Bacterial cellulose biosynthesis: diversity of operons, subunits, products, and functions.Trends Microbiol. 2015 Sep;23(9):545-57. doi: 10.1016/j.tim.2015.05.005. Epub 2015 Jun 12. Trends Microbiol. 2015. PMID: 26077867 Free PMC article. Review.
References
-
- Doblin MS, Kurek I, Jacob-Wilk D, Delmer DP. Cellulose biosynthesis in plants: From genes to rosettes. Plant Cell Physiol. 2002;43:1407–1420. - PubMed
-
- Kimura S, Kondo T. Recent progress in cellulose biosynthesis. J Plant Res. 2002;115:297–302. - PubMed
-
- Taylor NG. Cellulose biosynthesis and deposition in higher plants. New Phytol. 2008;179:239–252. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

