Bacterial Na+-translocating ferredoxin:NAD+ oxidoreductase
- PMID: 20921383
- PMCID: PMC2964206
- DOI: 10.1073/pnas.1010318107
Bacterial Na+-translocating ferredoxin:NAD+ oxidoreductase
Abstract
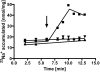
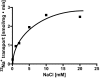
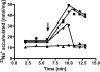
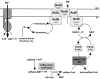
The anaerobic acetogenic bacterium Acetobacterium woodii carries out a unique type of Na(+)-motive, anaerobic respiration with caffeate as electron acceptor, termed "caffeate respiration." Central, and so far the only identified membrane-bound reaction in this respiration pathway, is a ferredoxin:NAD(+) oxidoreductase (Fno) activity. Here we show that inverted membrane vesicles of A. woodii couple electron transfer from reduced ferredoxin to NAD(+) with the transport of Na(+) from the outside into the lumen of the vesicles. Na(+) transport was electrogenic, and accumulation was inhibited by sodium ionophores but not protonophores, demonstrating a direct coupling of Fno activity to Na(+) transport. Results from inhibitor studies are consistent with the hypothesis that Fno activity coupled to Na(+) translocation is catalyzed by the Rnf complex, a membrane-bound, iron-sulfur and flavin-containing electron transport complex encoded by many bacterial and some archaeal genomes. Fno is a unique type of primary Na(+) pump and represents an early evolutionary mechanism of energy conservation that expands the redox range known to support life. In addition, it explains the lifestyle of many anaerobic bacteria and gives a mechanistic explanation for the enigma of the energetic driving force for the endergonic reduction of ferredoxin with NADH plus H(+) as reductant in a number of aerobic bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
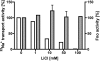
References
-
- Wood HG, Ragsdale SW, Pezacka E. The acetyl-CoA pathway of autotrophic growth. FEMS Microbiol Rev. 1986;39:345–362.
-
- Ljungdahl LG. In: Acetogenesis. Drake HL, editor. New York: Chapman & Hall; 1994. pp. 63–87.
-
- Fritz M, et al. An intermediate step in the evolution of ATPases: A hybrid F0V0 rotor in a bacterial Na+F1F0 ATP synthase. FEBS J. 2008;275:1999–2007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

