Development of the malaria parasite in the skin of the mammalian host
- PMID: 20921402
- PMCID: PMC2972976
- DOI: 10.1073/pnas.1009346107
Development of the malaria parasite in the skin of the mammalian host
Abstract
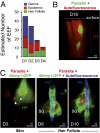
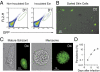
The first step of Plasmodium development in vertebrates is the transformation of the sporozoite, the parasite stage injected by the mosquito in the skin, into merozoites, the stage that invades erythrocytes and initiates the disease. The current view is that, in mammals, this stage conversion occurs only inside hepatocytes. Here, we document the transformation of sporozoites of rodent-infecting Plasmodium into merozoites in the skin of mice. After mosquito bite, ∼50% of the parasites remain in the skin, and at 24 h ∼10% are developing in the epidermis and the dermis, as well as in the immunoprivileged hair follicles where they can survive for weeks. The parasite developmental pathway in skin cells, although frequently abortive, leads to the generation of merozoites that are infective to erythrocytes and are released via merosomes, as typically observed in the liver. Therefore, during malaria in rodents, the skin is not just the route to the liver but is also the final destination for many inoculated parasites, where they can differentiate into merozoites and possibly persist.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Huff CG. Life cycle of malarial parasites. Annu Rev Microbiol. 1947;1:43–60.
-
- Shortt HE, Garnham PC. Pre-erythrocytic stage in mammalian malaria parasites. Nature. 1948;161:126. - PubMed
-
- Vanderberg JP. Plasmodium berghei exoerythrocytic forms develop only in the liver. Trans R Soc Trop Med Hyg. 1981;75:904–905. - PubMed
-
- Meis JF, Verhave JP. Exoerythrocytic development of malarial parasites. Adv Parasitol. 1988;27:1–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

