Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells
- PMID: 20921404
- PMCID: PMC2964233
- DOI: 10.1073/pnas.1011558107
Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells
Abstract
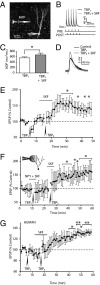
The mechanisms underlying memory formation in the hippocampal network remain a major unanswered aspect of neuroscience. Although high-frequency activity appears essential for plasticity, salience for memory formation is also provided by activity in ventral tegmental area (VTA) dopamine projections. Here, we report that activation of dopamine D1 receptors in dentate granule cells (DGCs) can preferentially increase dendritic excitability to both high-frequency afferent activity and high-frequency trains of backpropagating action potentials. Using whole-cell patch clamp recordings, calcium imaging, and neuropeptide Y to inhibit postsynaptic calcium influx, we found that activation of dendritic voltage-dependent calcium channels (VDCCs) is essential for dopamine-induced long-term potentiation (LTP), both in rat and human dentate gyrus (DG). Moreover, we demonstrate previously unreported spike-timing-dependent plasticity in the human hippocampus. These results suggest that when dopamine is released in the dentate gyrus with concurrent high-frequency activity there is an increased probability that synapses will be strengthened and reward-associated spatial memories will be formed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Dopamine D1 and D5 receptors modulate spike timing-dependent plasticity at medial perforant path to dentate granule cell synapses.J Neurosci. 2014 Nov 26;34(48):15888-97. doi: 10.1523/JNEUROSCI.2400-14.2014. J Neurosci. 2014. PMID: 25429131 Free PMC article.
-
Action potential-induced dendritic calcium dynamics correlated with synaptic plasticity in developing hippocampal pyramidal cells.J Neurophysiol. 1999 Oct;82(4):1993-9. doi: 10.1152/jn.1999.82.4.1993. J Neurophysiol. 1999. PMID: 10515989
-
Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites.Science. 1995 Apr 14;268(5208):297-300. doi: 10.1126/science.7716524. Science. 1995. PMID: 7716524
-
The role of postsynaptic calcium in the induction of long-term potentiation.Mol Neurobiol. 1991;5(2-4):289-95. doi: 10.1007/BF02935552. Mol Neurobiol. 1991. PMID: 1668390 Review.
-
Active dendrites, potassium channels and synaptic plasticity.Philos Trans R Soc Lond B Biol Sci. 2003 Apr 29;358(1432):667-74. doi: 10.1098/rstb.2002.1248. Philos Trans R Soc Lond B Biol Sci. 2003. PMID: 12740112 Free PMC article. Review.
Cited by
-
Dopaminergic inputs in the dentate gyrus direct the choice of memory encoding.Proc Natl Acad Sci U S A. 2016 Sep 13;113(37):E5501-10. doi: 10.1073/pnas.1606951113. Epub 2016 Aug 29. Proc Natl Acad Sci U S A. 2016. PMID: 27573822 Free PMC article.
-
Cognitive science theory-driven pharmacology elucidates the neurobiological basis of perception-motor integration.Commun Biol. 2022 Sep 6;5(1):919. doi: 10.1038/s42003-022-03864-1. Commun Biol. 2022. PMID: 36068298 Free PMC article. Clinical Trial.
-
Generation of silent synapses in dentate gyrus correlates with development of alcohol addiction.Neuropsychopharmacology. 2018 Sep;43(10):1989-1999. doi: 10.1038/s41386-018-0119-4. Epub 2018 Jun 15. Neuropsychopharmacology. 2018. PMID: 29967367 Free PMC article.
-
Cadmium bioaccumulates after acute exposure but has no effect on locomotion or shelter-seeking behaviour in the invasive green shore crab (Carcinus maenas).Conserv Physiol. 2017 Sep 28;5(1):cox057. doi: 10.1093/conphys/cox057. eCollection 2017. Conserv Physiol. 2017. PMID: 28979787 Free PMC article.
-
R-Modafinil exerts weak effects on spatial memory acquisition and dentate gyrus synaptic plasticity.PLoS One. 2017 Jun 23;12(6):e0179675. doi: 10.1371/journal.pone.0179675. eCollection 2017. PLoS One. 2017. PMID: 28644892 Free PMC article.
References
-
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. - PubMed
-
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006;16:716–729. - PubMed
-
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. - PubMed
-
- Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

