Candidate genes and mechanisms for 2-methoxyestradiol-mediated vasoprotection
- PMID: 20921434
- PMCID: PMC3023414
- DOI: 10.1161/HYPERTENSIONAHA.110.152298
Candidate genes and mechanisms for 2-methoxyestradiol-mediated vasoprotection
Erratum in
-
Correction.Hypertension. 2016 May;67(5):e21. doi: 10.1161/HYP.0000000000000045. Hypertension. 2016. PMID: 27075470 No abstract available.
Abstract
2-Methoxyestradiol (2-ME; estradiol metabolite) inhibits vascular smooth muscle cell (VSMC) growth and protects against atherosclerosis and vascular injury; however, the mechanisms by which 2-ME induces these actions remain obscure. To assess the impact of 2-ME on biochemical pathways regulating VSMC biology, we used high-density oligonucleotide microarrays to identify differentially expressed genes in cultured human female aortic VSMCs treated with 2-ME acutely (4 hours) or long term (30 hours). Both single gene analysis and Gene Set Enrichment Analysis revealed 2-ME-induced downregulation of genes involved in mitotic spindle assembly and function in VSMCs. Also, Gene Set Enrichment Analysis identified effects of 2-ME on genes regulating cell-cycle progression, cell migration/adhesion, vasorelaxation, inflammation, and cholesterol metabolism. Transcriptional changes were associated with changes in protein expression, including inhibition of cyclin D1, cyclin B1, cyclin-dependent kinase 6, cyclin-dependent kinase 4, tubulin polymerization, cholesterol and steroid synthesis, and upregulation of cyclooxygenase 2 and matrix metalloproteinase 1. Microarray data suggested that 2-ME may activate peroxisome proliferator-activated receptors (PPARs) in VSMCs, and 2-ME has structural similarities with rosiglitazone (PPARγ agonist). However, our finding of weak activation and lack of binding of 2-ME to PPARs suggests that 2-ME may modulate PPAR-associated genes via indirect mechanisms, potentially involving cyclooxygenase 2. Indeed, the antimitogenic effects of 2-ME at concentrations that do not inhibit tubulin polymerization were blocked by the PPAR antagonist GW9662 and the cyclooxygenase 2 inhibitor NS398. Finally, we demonstrated that 2-ME inhibited hypoxia-inducible factor 1α. Identification of candidate genes that are positively or negatively regulated by 2-ME provides important leads to investigate and better understand the mechanisms by which 2-ME induces its vasoprotective actions.
Figures
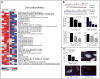



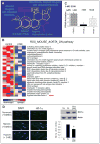
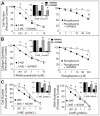
Comment in
-
Estrogen metabolomics: a physiologist's perspective.Hypertension. 2010 Nov;56(5):816-8. doi: 10.1161/HYPERTENSIONAHA.110.154385. Epub 2010 Oct 4. Hypertension. 2010. PMID: 20921431 Free PMC article. No abstract available.
References
-
- Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis and cancer: Common molecular pathways of disease development and progression. Ann NY Acad Sci. 2001;947:271–292. - PubMed
-
- Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression. New therapeutic target for vascular proliferative disease. Circulation. 1998;98:82–89. - PubMed
-
- Dubey RK, Imthurn B, Jackson EK. 2-Methoxyestradiol: a potential treatment for multiple proliferative disorders. Endocrinology. 2007;148:4125–4127. - PubMed
-
- Mooberry SL. New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Current Opinion in Oncology. 2003;15:425–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

