In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes
- PMID: 20921443
- PMCID: PMC2981142
- DOI: 10.1161/CIRCULATIONAHA.109.879338
In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes
Abstract
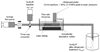
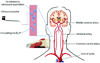
Background: Ischemia-related neurological injury is a primary cause of stroke disability. Studies have demonstrated that xenon (Xe) may have potential as an effective and nontoxic neuroprotectant. Xe delivery is, however, hampered by lack of suitable administration methods. We have developed a pressurization-freeze method to encapsulate Xe into echogenic liposomes (Xe-ELIP) and have modulated local gas release with transvascular ultrasound exposure.
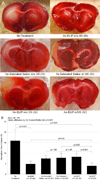
Methods and results: Fifteen microliters of Xe were encapsulated into each 1 mg of liposomes (70% Xe and 30% argon). Xe delivery from Xe-ELIP into cells and consequent neuroprotective effects were evaluated with oxygen/glucose-deprived and control neuronal cells in vitro. Xe-ELIP were administered into Sprague-Dawley rats intravenously or intra-arterially after right middle cerebral artery occlusion. One-megahertz low-amplitude (0.18 MPa) continuous wave ultrasound directed onto the internal carotid artery triggered Xe release from circulating Xe-ELIP. Effects of Xe delivery on ischemia-induced neurological injury and disability were evaluated. Xe-ELIP delivery to oxygen/glucose-deprived neuronal cells improved cell viability in vitro and resulted in a 48% infarct volume decrease in vivo. Intravenous Xe-ELIP administration in combination with the ultrasound directed onto the carotid artery enhanced local Xe release from circulating Xe-ELIP and demonstrated 75% infarct volume reduction. This was comparable to the effect after intra-arterial administration. Behavioral tests on limb placement and grid and beam walking correlated with infarct reduction.
Conclusions: This novel methodology may provide a noninvasive strategy for ultrasound-enhanced local therapeutic gas delivery for cerebral ischemia-related injury while minimizing systemic side effects.
Conflict of interest statement
There are no conflicts to disclose.
Figures





Similar articles
-
Therapeutic time window and dose dependence of xenon delivered via echogenic liposomes for neuroprotection in stroke.CNS Neurosci Ther. 2013 Oct;19(10):773-84. doi: 10.1111/cns.12159. Epub 2013 Aug 24. CNS Neurosci Ther. 2013. PMID: 23981565 Free PMC article.
-
Delivery of xenon-containing echogenic liposomes inhibits early brain injury following subarachnoid hemorrhage.Sci Rep. 2018 Jan 11;8(1):450. doi: 10.1038/s41598-017-18914-6. Sci Rep. 2018. PMID: 29323183 Free PMC article.
-
Gas chromatography/mass spectrometry measurement of xenon in gas-loaded liposomes for neuroprotective applications.Rapid Commun Mass Spectrom. 2017 Jan 15;31(1):1-8. doi: 10.1002/rcm.7749. Rapid Commun Mass Spectrom. 2017. PMID: 27689777 Free PMC article.
-
Enhanced Cerebroprotection of Xenon-Loaded Liposomes in Combination with rtPA Thrombolysis for Embolic Ischemic Stroke.Biomolecules. 2023 Aug 16;13(8):1256. doi: 10.3390/biom13081256. Biomolecules. 2023. PMID: 37627321 Free PMC article.
-
Neuroprotection by mesenchymal stem cell (MSC) administration is enhanced by local cooling infusion (LCI) in ischemia.Brain Res. 2019 Dec 1;1724:146406. doi: 10.1016/j.brainres.2019.146406. Epub 2019 Aug 24. Brain Res. 2019. PMID: 31454517 Review.
Cited by
-
Cavitation Emissions Nucleated by Definity Infused through an EkoSonic Catheter in a Flow Phantom.Ultrasound Med Biol. 2021 Mar;47(3):693-709. doi: 10.1016/j.ultrasmedbio.2020.10.010. Epub 2021 Jan 7. Ultrasound Med Biol. 2021. PMID: 33349516 Free PMC article.
-
Ultrasound enhanced matrix metalloproteinase-9 triggered release of contents from echogenic liposomes.Mol Pharm. 2012 Sep 4;9(9):2554-64. doi: 10.1021/mp300165s. Epub 2012 Aug 15. Mol Pharm. 2012. PMID: 22849291 Free PMC article.
-
Effects of inhaled low-concentration xenon gas on naltrexone-precipitated withdrawal symptoms in morphine-dependent mice.Drug Alcohol Depend. 2024 Feb 1;255:110967. doi: 10.1016/j.drugalcdep.2023.110967. Epub 2023 Sep 19. Drug Alcohol Depend. 2024. PMID: 38150894 Free PMC article.
-
Encapsulated microbubbles and echogenic liposomes for contrast ultrasound imaging and targeted drug delivery.Comput Mech. 2014 Mar;53(3):413-435. doi: 10.1007/s00466-013-0962-4. Comput Mech. 2014. PMID: 26097272 Free PMC article.
-
Stimuli-responsive nanocarriers for drug delivery.Nat Mater. 2013 Nov;12(11):991-1003. doi: 10.1038/nmat3776. Nat Mater. 2013. PMID: 24150417 Review.
References
-
- Suwanwela NC, Eusattasak N, Phanthumchinda K, Piravej K, Locharoenkul C. Combination of acute stroke unit and short-term stroke ward with early supported discharge decreases mortality and complications after acute ischemic stroke. J Med Assoc Thai. 2007;90:1089–1096. - PubMed
-
- Goldstein LB, Rothwell PM. Primary prevention and health services delivery. Stroke. 2007;38:222–224. - PubMed
-
- Wagner KR, Jauch EC. Extending the window for acute stroke treatment: thrombolytics plus CNS protective therapies. Exp Neurol. 2004;188:195–199. - PubMed
-
- Catarzi D, Colotta V, Varano F. Competitive Gly/NMDA receptor antagonists. Curr Top Med Chem. 2006;6:809–821. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

