Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma
- PMID: 20921456
- PMCID: PMC3020706
- DOI: 10.1200/JCO.2010.30.3545
Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma
Erratum in
- J Clin Oncol. 2010 Dec 20;28(36):5351. Panandiker, Atmaram Pai [corrected to Pai Panandiker, Atmaram S]
Abstract
Purpose: To evaluate the safety, maximum-tolerated dose, pharmacokinetics, and pharmacodynamics of vandetanib, an oral vascular endothelial growth factor receptor 2 (VEGFR2) and epidermal growth factor receptor inhibitor, administered once daily during and after radiotherapy in children with newly diagnosed diffuse intrinsic pontine glioma.
Patients and methods: Radiotherapy was administered as 1.8-Gy fractions (total cumulative dose of 54 Gy). Vandetanib was administered concurrently with radiotherapy for a maximum of 2 years. Dose-limiting toxicities (DLTs) were evaluated during the first 6 weeks of therapy. Pharmacokinetic studies were obtained for all patients. Plasma angiogenic factors and VEGFR2 phosphorylation in mononuclear cells were analyzed before and during therapy.
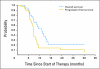
Results: Twenty-one patients were administered 50 (n = 3), 65 (n = 3), 85 (n = 3), 110 (n = 6), and 145 mg/m(2) (n = 6) of vandetanib. Only one patient developed DLT (grade 3 diarrhea) at dosage level 5. An expanded cohort of patients were treated at dosage levels 4 (n = 10) and 5 (n = 4); two patients developed grade 4 hypertension and posterior reversible encephalopathy syndrome while also receiving high-dose dexamethasone. Despite significant interpatient variability, exposure to vandetanib increased with higher dosage levels. The bivariable analysis of vascular endothelial growth factor (VEGF) before and during therapy showed that patients with higher levels of VEGF before therapy had a longer progression-free survival (PFS; P = .022), whereas patients with increases in VEGF during treatment had a shorter PFS (P = .0015). VEGFR2 phosphorylation was inhibited on day 8 or 29 of therapy compared with baseline (P = .039).
Conclusion: The recommended phase II dose of vandetanib in children is 145 mg/m(2) per day. Close monitoring and management of hypertension is required, particularly for patients receiving corticosteroids.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Similar articles
-
Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma.Clin Cancer Res. 2013 Jun 1;19(11):3050-8. doi: 10.1158/1078-0432.CCR-13-0306. Epub 2013 Mar 27. Clin Cancer Res. 2013. PMID: 23536435 Free PMC article. Clinical Trial.
-
A phase I/II trial of vandetanib for patients with recurrent malignant glioma.Neuro Oncol. 2012 Dec;14(12):1519-26. doi: 10.1093/neuonc/nos265. Epub 2012 Oct 25. Neuro Oncol. 2012. PMID: 23099652 Free PMC article. Clinical Trial.
-
Pharmacokinetics and tolerability of vandetanib in Chinese patients with solid, malignant tumors: an open-label, phase I, rising multiple-dose study.Clin Ther. 2011 Mar;33(3):315-27. doi: 10.1016/j.clinthera.2011.04.005. Clin Ther. 2011. PMID: 21600385 Clinical Trial.
-
Vandetanib: in medullary thyroid cancer.Drugs. 2012 Jul 9;72(10):1423-36. doi: 10.2165/11209300-000000000-00000. Drugs. 2012. PMID: 22715896 Review.
-
[Diffuse intrinsic brain stem glioma in children: current treatment and future directions].Arch Pediatr. 2010 Feb;17(2):159-65. doi: 10.1016/j.arcped.2009.11.016. Epub 2009 Dec 16. Arch Pediatr. 2010. PMID: 20018494 Review. French.
Cited by
-
Defining Optimal Target Volumes of Conformal Radiation Therapy for Diffuse Intrinsic Pontine Glioma.Int J Radiat Oncol Biol Phys. 2020 Mar 15;106(4):838-847. doi: 10.1016/j.ijrobp.2019.11.020. Epub 2019 Nov 27. Int J Radiat Oncol Biol Phys. 2020. PMID: 31785339 Free PMC article.
-
Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries.J Clin Oncol. 2018 Jul 1;36(19):1963-1972. doi: 10.1200/JCO.2017.75.9308. Epub 2018 May 10. J Clin Oncol. 2018. PMID: 29746225 Free PMC article.
-
Repurposing Vandetanib plus Everolimus for the Treatment of ACVR1-Mutant Diffuse Intrinsic Pontine Glioma.Cancer Discov. 2022 Feb;12(2):416-431. doi: 10.1158/2159-8290.CD-20-1201. Epub 2021 Sep 22. Cancer Discov. 2022. PMID: 34551970 Free PMC article.
-
Co-administration strategy to enhance brain accumulation of vandetanib by modulating P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp1/Abcg2) mediated efflux with m-TOR inhibitors.Int J Pharm. 2012 Sep 15;434(1-2):306-14. doi: 10.1016/j.ijpharm.2012.05.028. Epub 2012 May 23. Int J Pharm. 2012. PMID: 22633931 Free PMC article.
-
Autophagy inhibition induces enhanced proapoptotic effects of ZD6474 in glioblastoma.Br J Cancer. 2013 Jul 9;109(1):164-71. doi: 10.1038/bjc.2013.306. Epub 2013 Jun 25. Br J Cancer. 2013. PMID: 23799852 Free PMC article.
References
-
- Hargrave D, Bartels U, Bouffet E, et al. Diffuse brainstem glioma in children: Critical review of clinical trials. Lancet Oncol. 2006;7:241–248. - PubMed
-
- Zarghooni M, Bartels U, Lee E, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28:1337–1344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

