Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma
- PMID: 20921459
- PMCID: PMC3020702
- DOI: 10.1200/JCO.2010.28.6963
Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma
Abstract
Purpose: Immunologic targeting of tumor-specific gene mutations may allow precise eradication of neoplastic cells without toxicity. Epidermal growth factor receptor variant III (EGFRvIII) is a constitutively activated and immunogenic mutation not expressed in normal tissues but widely expressed in glioblastoma multiforme (GBM) and other neoplasms.
Patients and methods: A phase II, multicenter trial was undertaken to assess the immunogenicity of an EGFRvIII-targeted peptide vaccine and to estimate the progression-free survival (PFS) and overall survival (OS) of vaccinated patients with newly diagnosed EGFRvIII-expressing GBM with minimal residual disease. Intradermal vaccinations were given until toxicity or tumor progression was observed. Sample size was calculated to differentiate between PFS rates of 20% and 40% 6 months after vaccination.
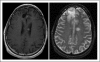
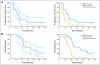
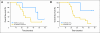
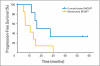
Results: There were no symptomatic autoimmune reactions. The 6-month PFS rate after vaccination was 67% (95% CI, 40% to 83%) and after diagnosis was 94% (95% CI, 67% to 99%; n = 18). The median OS was 26.0 months (95% CI, 21.0 to 47.7 months). After adjustment for age and Karnofsky performance status, the OS of vaccinated patients was greater than that observed in a control group matched for eligibility criteria, prognostic factors, and temozolomide treatment (hazard ratio, 5.3; P = .0013; n = 17). The development of specific antibody (P = .025) or delayed-type hypersensitivity (P = .03) responses to EGFRvIII had a significant effect on OS. At recurrence, 82% (95% CI, 48% to 97%) of patients had lost EGFRvIII expression (P < .001).
Conclusion: EGFRvIII-targeted vaccination in patients with GBM warrants investigation in a phase III, randomized trial.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Cancer vaccines in glioma: how to balance the challenges of small trials, efficiency, and potential adverse events.J Clin Oncol. 2010 Nov 1;28(31):4670-3. doi: 10.1200/JCO.2010.32.1117. Epub 2010 Oct 4. J Clin Oncol. 2010. PMID: 20921460 No abstract available.
-
Immunotherapy: A promising vaccine for glioblastoma multiforme.Nat Rev Clin Oncol. 2011 Jan;8(1):4. doi: 10.1038/nrclinonc.2010.201. Nat Rev Clin Oncol. 2011. PMID: 21218529 No abstract available.
-
How fine a slice: treatment of newly diagnosed glioblastoma with an epidermal growth factor receptor variant III peptide vaccine.J Clin Oncol. 2011 Jun 10;29(17):e517-8; author reply e519-20. doi: 10.1200/JCO.2010.34.0588. Epub 2011 May 9. J Clin Oncol. 2011. PMID: 21555685 No abstract available.
-
Immunotherapy for glioblastoma: the devil is in the details.J Clin Oncol. 2011 Aug 1;29(22):3105; author reply 3105-6. doi: 10.1200/JCO.2011.34.9019. Epub 2011 Jun 27. J Clin Oncol. 2011. PMID: 21709193 No abstract available.
References
-
- Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818–822. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–1466. - PubMed
-
- Ge H, Gong X, Tang CK. Evidence of high incidence of EGFRvIII expression and coexpression with EGFR in human invasive breast cancer by laser capture microdissection and immunohistochemical analysis. Int J Cancer. 2002;98:357–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

