Identification of an autophagy defect in smokers' alveolar macrophages
- PMID: 20921532
- PMCID: PMC3057181
- DOI: 10.4049/jimmunol.1001603
Identification of an autophagy defect in smokers' alveolar macrophages
Abstract
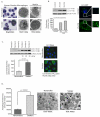
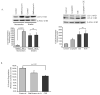
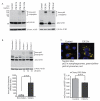
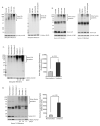
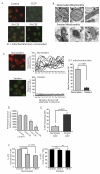
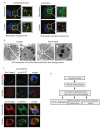
Alveolar macrophages are essential for clearing bacteria from the alveolar surface and preventing microbe-induced infections. It is well documented that smokers have an increased incidence of infections, in particular lung infections. Alveolar macrophages accumulate in smokers' lungs, but they have a functional immune deficit. In this study, we identify an autophagy defect in smokers' alveolar macrophages. Smokers' alveolar macrophages accumulate both autophagosomes and p62, a marker of autophagic flux. The decrease in the process of autophagy leads to impaired protein aggregate clearance, dysfunctional mitochondria, and defective delivery of bacteria to lysosomes. This study identifies the autophagy pathway as a potential target for interventions designed to decrease infection rates in smokers and possibly in individuals with high environmental particulate exposure.
Figures

References
-
- Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. The New England journal of medicine. 1974;291:755–758. - PubMed
-
- Green GM. Cigarette smoke: protection of alveolar macrophages by glutathione and cysteine. Science (New York, N.Y. 1968;162:810–811. - PubMed
-
- Laurenzi GA, Guarneri JJ, Endriga RB, Carey JP. Clearance of Bacteria by the Lower Respiratory Tract. Science (New York, N.Y. 1963;142:1572–1573. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

