Placental growth factor mediates aldosterone-dependent vascular injury in mice
- PMID: 20921624
- PMCID: PMC2964966
- DOI: 10.1172/JCI40205
Placental growth factor mediates aldosterone-dependent vascular injury in mice
Abstract
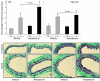
In clinical trials, aldosterone antagonists reduce cardiovascular ischemia and mortality by unknown mechanisms. Aldosterone is a steroid hormone that signals through renal mineralocorticoid receptors (MRs) to regulate blood pressure. MRs are expressed and regulate gene transcription in human vascular cells, suggesting that aldosterone might have direct vascular effects. Using gene expression profiling, we identify the pro-proliferative VEGF family member placental growth factor (PGF) as an aldosterone-regulated vascular MR target gene in mice and humans. Aldosterone-activated vascular MR stimulated Pgf gene transcription and increased PGF protein expression and secretion in the mouse vasculature. In mouse vessels with endothelial damage and human vessels from patients with atherosclerosis, aldosterone enhanced expression of PGF and its receptor, FMS-like tyrosine kinase 1 (Flt1). In atherosclerotic human vessels, MR antagonists inhibited PGF expression. In vivo, aldosterone infusion augmented vascular remodeling in mouse carotids following wire injury, an effect that was lost in Pgf-/- mice. In summary, we have identified PGF as what we believe to be a novel downstream target of vascular MR that mediates aldosterone augmentation of vascular injury. These findings suggest a non-renal mechanism for the vascular protective effects of aldosterone antagonists in humans and support targeting the vascular aldosterone/MR/PGF/Flt1 pathway as a therapeutic strategy for ischemic cardiovascular disease.
Figures
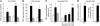
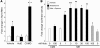




Similar articles
-
Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors.Arterioscler Thromb Vasc Biol. 2014 Feb;34(2):355-64. doi: 10.1161/ATVBAHA.113.302854. Epub 2013 Dec 5. Arterioscler Thromb Vasc Biol. 2014. PMID: 24311380 Free PMC article.
-
Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion.Circ Res. 2008 Jun 6;102(11):1359-67. doi: 10.1161/CIRCRESAHA.108.174235. Epub 2008 May 8. Circ Res. 2008. PMID: 18467630 Free PMC article.
-
Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1.Nat Med. 2003 Jul;9(7):936-43. doi: 10.1038/nm884. Nat Med. 2003. PMID: 12796773
-
Vascular Consequences of Aldosterone Excess and Mineralocorticoid Receptor Antagonism.Curr Hypertens Rev. 2017;13(1):46-56. doi: 10.2174/1573402113666170228151402. Curr Hypertens Rev. 2017. PMID: 28245785 Review.
-
Does Aldosterone Play a Significant Role for Regulation of Vascular Tone?J Cardiovasc Pharmacol. 2016 Jul;68(1):1-10. doi: 10.1097/FJC.0000000000000345. J Cardiovasc Pharmacol. 2016. PMID: 26657712 Review.
Cited by
-
Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis.Nat Rev Nephrol. 2013 Aug;9(8):459-69. doi: 10.1038/nrneph.2013.110. Epub 2013 Jun 18. Nat Rev Nephrol. 2013. PMID: 23774812 Free PMC article. Review.
-
Placental Growth Factor as a Predictor of Cardiovascular Events in Patients with CKD from the NARA-CKD Study.J Am Soc Nephrol. 2015 Nov;26(11):2871-81. doi: 10.1681/ASN.2014080772. Epub 2015 Mar 18. J Am Soc Nephrol. 2015. PMID: 25788536 Free PMC article.
-
Smooth Muscle Cell Notch2 Is Not Required for Atherosclerotic Plaque Formation in ApoE Null Mice.J Vasc Res. 2022;59(5):261-274. doi: 10.1159/000525258. Epub 2022 Jul 7. J Vasc Res. 2022. PMID: 35797968 Free PMC article.
-
Mineralocorticoid receptor antagonism attenuates experimental pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2013 May 15;304(10):L678-88. doi: 10.1152/ajplung.00300.2012. Epub 2013 Mar 1. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23457185 Free PMC article.
-
Loss of placental growth factor ameliorates maternal hypertension and preeclampsia in mice.J Clin Invest. 2018 Nov 1;128(11):5008-5017. doi: 10.1172/JCI99026. Epub 2018 Oct 8. J Clin Invest. 2018. PMID: 30179860 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

