Specialized roles for cysteine cathepsins in health and disease
- PMID: 20921628
- PMCID: PMC2947230
- DOI: 10.1172/JCI42918
Specialized roles for cysteine cathepsins in health and disease
Abstract
Cathepsins were originally identified as proteases that act in the lysosome. Recent work has uncovered nontraditional roles for cathepsins in the extracellular space as well as in the cytosol and nucleus. There is strong evidence that subspecialized and compartmentalized cathepsins participate in many physiologic and pathophysiologic cellular processes, in which they can act as both digestive and regulatory proteases. In this review, we discuss the transcriptional and translational control of cathepsin expression, the regulation of intracellular sorting of cathepsins, and the structural basis of cathepsin activation and inhibition. In particular, we highlight the emerging roles of various cathepsin forms in disease, particularly those of the cardiac and renal systems.
Figures


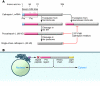
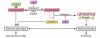
References
-
- Willstätter R, Bamann E. Über die Proteasen der Magenschleimhaut. Erste Abhandlung über die Enzyme der Leukozyten. Hoppe-Seyler’s Z Physiol Chem. 1929;180(1–3):127–143.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

