Sustained long-term antiviral maintenance therapy in HCV/HIV-coinfected patients (SLAM-C)
- PMID: 20921898
- PMCID: PMC3017670
- DOI: 10.1097/QAI.0b013e3181f6d916
Sustained long-term antiviral maintenance therapy in HCV/HIV-coinfected patients (SLAM-C)
Abstract
Background: Hepatitis C virus (HCV)/HIV coinfection treatment is suboptimal with low sustained viral response rates to standard therapies. A multicenter randomized clinical trial designed to assess the efficacy/safety of pegylated interferon maintenance therapy was performed by the National Institutes of Health-funded AIDS Clinical Trials Group network.
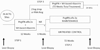
Methods: HCV treatment-naive and nonresponding interferon-experienced subjects with confirmed HCV and HIV, CD4 >200 cells per cubic millimeter, and at least stage 1 fibrosis were enrolled and treated for 12 weeks with pegylated interferon alfa 2a 180 mcg per week (PEG) + weight-based ribavirin to determine response status. Nonresponder subjects (failure to clear HCV RNA or achieve 2-log drop) underwent liver biopsy and were randomized to receive full dose PEG or observation only for 72 weeks. Paired biopsies were evaluated by a central pathologist.
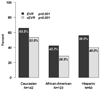
Results: Three hundred thirty subjects were enrolled; median age was 48 years; 43% white, 37% black, non-Hispanic; 83% male; CD4+ 498 cells per cubic millimeter; 32% were interferon experienced; 74% had entry HIV RNA <50 copies per milliliter. early virologic responder was observed in 55.9% and 42.5% achieved complete Early Viral Response (cEVR). A planned interim analysis of occurred when 84 subjects were randomized. With data on 40 paired biopsies available, a safety monitoring board stopped the trial due to lack of fibrosis progression (median = 0 Metavir units/year) in the observation arm.
Conclusions: Lack of fibrotic progression in the control arm was unexpected and may represent a short-term PEG/ribavirin therapy effect, high levels of HIV viral suppression, and use of antiretroviral regimens that may be less toxic than prior generations of therapy.
Trial registration: ClinicalTrials.gov NCT00078403.
Figures



Similar articles
-
Response predictors and clinical benefits of hepatitis C retreatment with pegylated interferon and ribavirin in HIV/HCV coinfection.Ann Hepatol. 2013 Mar-Apr;12(2):228-35. Ann Hepatol. 2013. PMID: 23396734
-
Addition of nitazoxanide to PEG-IFN and ribavirin to improve HCV treatment response in HIV-1 and HCV genotype 1 coinfected persons naïve to HCV therapy: results of the ACTG A5269 trial.HIV Clin Trials. 2013 Nov-Dec;14(6):274-83. doi: 10.1310/hct1406-274. HIV Clin Trials. 2013. PMID: 24334180 Free PMC article. Clinical Trial.
-
Predictive value of early virologic response in HIV/hepatitis C virus-coinfected patients treated with an interferon-based regimen plus ribavirin.J Acquir Immune Defic Syndr. 2007 Feb 1;44(2):174-8. doi: 10.1097/QAI.0b013e31802b812d. J Acquir Immune Defic Syndr. 2007. PMID: 17106276 Clinical Trial.
-
Efficacy of early treatment of acute hepatitis C infection with pegylated interferon and ribavirin in HIV-infected patients.AIDS. 2006 May 12;20(8):1157-61. doi: 10.1097/01.aids.0000226956.02719.fd. AIDS. 2006. PMID: 16691067 Review.
-
Simeprevir with pegylated interferon alfa 2a plus ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a meta-analysis and historical comparison.BMC Infect Dis. 2016 Jan 11;16:10. doi: 10.1186/s12879-015-1311-3. BMC Infect Dis. 2016. PMID: 26753774 Free PMC article. Review.
Cited by
-
Characterization of HCV NS3 Protease Variants in HCV/HIV-Coinfected Patients by Ultra-Deep Sequence Analysis: Relationship with Hepatic Fibrosis.J Acquir Immune Defic Syndr. 2017 Mar 1;74(3):353-358. doi: 10.1097/QAI.0000000000001256. J Acquir Immune Defic Syndr. 2017. PMID: 27898525 Free PMC article.
-
Extended therapy with pegylated interferon and weight-based ribavirin for HCV-HIV coinfected patients.HIV Clin Trials. 2012 Mar-Apr;13(2):70-82. doi: 10.1310/hct1302-70. HIV Clin Trials. 2012. PMID: 22510354 Free PMC article. Clinical Trial.
-
In HIV/hepatitis C virus co-infected patients, higher 25-hydroxyvitamin D concentrations were not related to hepatitis C virus treatment responses but were associated with ritonavir use.Am J Clin Nutr. 2013 Aug;98(2):423-9. doi: 10.3945/ajcn.112.048785. Epub 2013 Jun 5. Am J Clin Nutr. 2013. PMID: 23739141 Free PMC article.
-
Impact of peginterferon alpha and ribavirin treatment on lipid profiles and insulin resistance in Hepatitis C virus/HIV-coinfected persons: the AIDS Clinical Trials Group A5178 Study.Clin Infect Dis. 2012 Sep;55(5):631-8. doi: 10.1093/cid/cis463. Epub 2012 May 4. Clin Infect Dis. 2012. PMID: 22563020 Free PMC article. Clinical Trial.
-
Chronic liver disease in the Hispanic population of the United States.Clin Gastroenterol Hepatol. 2011 Oct;9(10):834-41; quiz e109-10. doi: 10.1016/j.cgh.2011.04.027. Epub 2011 May 12. Clin Gastroenterol Hepatol. 2011. PMID: 21628000 Free PMC article. Review.
References
-
- Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–1058. - PubMed
-
- Di Martino V, Rufat P, Boyer N, et al. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology. 2001 Dec;34(6):1193–1199. - PubMed
-
- Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32(3):492–497. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI069432/AI/NIAID NIH HHS/United States
- U01 AI069415/AI/NIAID NIH HHS/United States
- U01 AI069511/AI/NIAID NIH HHS/United States
- AI069423-03/AI/NIAID NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- U01 AI069502/AI/NIAID NIH HHS/United States
- U01 AI069513/AI/NIAID NIH HHS/United States
- P20 RR 11126/RR/NCRR NIH HHS/United States
- K24 DA034621/DA/NIDA NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- AI050410/AI/NIAID NIH HHS/United States
- AI069556/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069513/AI/NIAID NIH HHS/United States
- M01 RR000096/RR/NCRR NIH HHS/United States
- M01 RR000046/RR/NCRR NIH HHS/United States
- AI46370/AI/NIAID NIH HHS/United States
- U01 AI027661/AI/NIAID NIH HHS/United States
- K24 DK078772/DK/NIDDK NIH HHS/United States
- U0I AI069415-03/AI/NIAID NIH HHS/United States
- U54-RR023561/RR/NCRR NIH HHS/United States
- AI-69419/AI/NIAID NIH HHS/United States
- 3-UO1AI046376-05S4/AI/NIAID NIH HHS/United States
- P20 RR011126/RR/NCRR NIH HHS/United States
- U01 AI069465/AI/NIAID NIH HHS/United States
- 5-MO1RR00044/RR/NCRR NIH HHS/United States
- UL1 RR024996/RR/NCRR NIH HHS/United States
- UOI AI 069472/AI/NIAID NIH HHS/United States
- IUO1A1069472/PHS HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- AI069532/AI/NIAID NIH HHS/United States
- M01 RR000865/RR/NCRR NIH HHS/United States
- RR000046-48/RR/NCRR NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- U01AI069494/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- U01 AI069556/AI/NIAID NIH HHS/United States
- UM1 AI069428/AI/NIAID NIH HHS/United States
- AI069513/AI/NIAID NIH HHS/United States
- U01AI06949S-02/AI/NIAID NIH HHS/United States
- AI54907/AI/NIAID NIH HHS/United States
- U01 AI069428/AI/NIAID NIH HHS/United States
- U01A106942/PHS HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- P30 AI054907/AI/NIAID NIH HHS/United States
- A1069472/PHS HHS/United States
- U01 AI38858/AI/NIAID NIH HHS/United States
- AI27661/AI/NIAID NIH HHS/United States
- AI069439/AI/NIAID NIH HHS/United States
- AI069428/AI/NIAID NIH HHS/United States
- M01 RR000044/RR/NCRR NIH HHS/United States
- AI34853/AI/NIAID NIH HHS/United States
- U01AI069472-03/AI/NIAID NIH HHS/United States
- U01 AI046376/AI/NIAID NIH HHS/United States
- AI069452/AI/NIAID NIH HHS/United States
- M01RR00096/RR/NCRR NIH HHS/United States
- U01 AI069450/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI046370/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- AI27665/AI/NIAID NIH HHS/United States
- AI69465/AI/NIAID NIH HHS/United States
- U01 AI034853/AI/NIAID NIH HHS/United States
- 5UO1AI069502-03/AI/NIAID NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- R01 DA016065/DA/NIDA NIH HHS/United States
- 5U01AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069556/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- M01 RR000032/RR/NCRR NIH HHS/United States
- U01AI0695/AI/NIAID NIH HHS/United States
- M01RR-00032/RR/NCRR NIH HHS/United States
- M01-RR00865/RR/NCRR NIH HHS/United States
- U01 AI025859/AI/NIAID NIH HHS/United States
- UM1 AI069450/AI/NIAID NIH HHS/United States
- AI69450/AI/NIAID NIH HHS/United States
- K24 DK070528/DK/NIDDK NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- RR025780/RR/NCRR NIH HHS/United States
- U01AI069511-02/AI/NIAID NIH HHS/United States
- 5-MO1 RR00044/RR/NCRR NIH HHS/United States
- AI25859/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- AI69432/AI/NIAID NIH HHS/United States
- U01 AI069452/AI/NIAID NIH HHS/United States
- Z01 AI000695/ImNIH/Intramural NIH HHS/United States
- U01 AI027665/AI/NIAID NIH HHS/United States
- AI069471/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

