Genome-wide association study of antipsychotic-induced QTc interval prolongation
- PMID: 20921969
- PMCID: PMC3388904
- DOI: 10.1038/tpj.2010.76
Genome-wide association study of antipsychotic-induced QTc interval prolongation
Abstract
QT prolongation is associated with increased risk of cardiac arrhythmias. Identifying the genetic variants that mediate antipsychotic-induced prolongation may help to minimize this risk, which might prevent the removal of efficacious drugs from the market. We performed candidate gene analysis and five drug-specific genome-wide association studies (GWASs) with 492K single-nucleotide polymorphisms to search for genetic variation mediating antipsychotic-induced QT prolongation in 738 schizophrenia patients from the Clinical Antipsychotic Trial of Intervention Effectiveness study. Our candidate gene study suggests the involvement of NOS1AP and NUBPL (P-values=1.45 × 10(-05) and 2.66 × 10(-13), respectively). Furthermore, our top GWAS hit achieving genome-wide significance, defined as a Q-value <0.10 (P-value=1.54 × 10(-7), Q-value=0.07), located in SLC22A23, mediated the effects of quetiapine on prolongation. SLC22A23 belongs to a family of organic ion transporters that shuttle a variety of compounds, including drugs, environmental toxins and endogenous metabolites, across the cell membrane. This gene is expressed in the heart and is integral in mouse heart development. The genes mediating antipsychotic-induced QT prolongation partially overlap with the genes affecting normal QT interval variation. However, some genes may also be unique for drug-induced prolongation. This study demonstrates the potential of GWAS to discover genes and pathways that mediate antipsychotic-induced QT prolongation.
Conflict of interest statement
Dr Sullivan reports receiving research funding from Eli Lilly in connection with this project. Dr. Stroup reports receiving consulting funds from Janssen and Lilly. Dr. Perkins reports having received research funding from AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Otsuka Pharmaceutical Co. Ltd, Eli Lilly and Co., Janssen Pharmaceutica Products, and Pfizer Inc.; and consulting and educational fees from AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Eli Lilly and Co., Janssen Pharmaceuticals, GlaxoSmithKline, Forest Labs, Pfizer Inc and Shire. Dr Lieberman reports having received research funding from AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, GlaxoSmithKline, Janssen Pharmaceutica, Wyeth, Merck, Forest Labs, Allon and Pfizer; and has participated in research, consulting, advisory board or DSMB activities from Chephalon, Eli Lilly, Pfizer, Bioline, Lilly, AstraZeneca, Forest Labs, GlaxoSmithKline, Janssen Pharmaceutica, Otsuka, Wyeth and Solvay; and a patent with Repligen. Drs. Aberg, McClay, Liu, Adkins, Bukszár, Jia, Zhao and van den Oord report no financial relationships with commercial interests and have nothing to disclose.
Figures
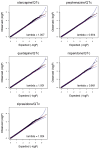
References
-
- Viskin S, Justo D, Halkin A, Zeltser D. Long QT syndrome caused by noncardiac drugs. Prog Cardiovasc Dis. 2003;45(5):415–427. - PubMed
-
- Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore) 2003;82(4):282–290. - PubMed
-
- Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58 (12):1161–1167. - PubMed
-
- Kane JM, Marder SR. Psychopharmacologic treatment of schizophrenia. Schizophr Bull. 1993;19(2):287–302. - PubMed
-
- Kane JM, McGlashan TH. Treatment of schizophrenia. Lancet. 1995;346(8978):820–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

