Inhibition of Hypoxia-Induced Cell Motility by p16 in MDA-MB-231 Breast Cancer Cells
- PMID: 20922054
- PMCID: PMC2948217
- DOI: 10.7150/jca.1.126
Inhibition of Hypoxia-Induced Cell Motility by p16 in MDA-MB-231 Breast Cancer Cells
Abstract
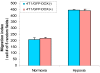
Our previous studies indicated that p16 suppresses breast cancer angiogenesis and metastasis, and downregulates VEGF gene expression by neutralizing the transactivation of the VEGF transcriptional factor HIF-1α. Hypoxia stimulates tumor malignant progression and induces HIF-1α. Because p16 neutralizes effect of HIF-1α and attenuates tumor metastatic progression, we intended to investigate whether p16 directly affects one or more aspects of the malignant process such as adhesion and migration of breast cancer cells. To approach this aim, MDA-MB-231 and other breast cancer cells stably transfected with Tet-on inducible p16 were used to study the p16 effect on growth, adhesion and migration of the cancer cells. We found that p16 inhibits breast cancer cell proliferation and migration, but has no apparent effect on cell adhesion. Importantly, p16 inhibits hypoxia-induced cell migration in breast cancer in parallel with its inhibition of HIF-1α transactivation activity. This study suggests that p16's ability to suppress tumor metastasis may be partially resulted from p16's inhibition on cell migration, in addition to its known functions on inhibition of cell proliferation, angiogenesis and induction of apoptosis.
Keywords: HIF-1a; breast cancer; hypoxia; migration/motility; p16.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures






References
-
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. - PubMed
-
- Roth U, Curth K, Unterman TG, Kietzmann T. The transcription factors HIF-1 and HNF-4 and the coactivator p300 are involved in insulin-regulated glucokinase gene expression via the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2004;279:2623–31. - PubMed
-
- Dachs GU, Patterson AV, Firth JD. et al.Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3:515–20. - PubMed
-
- Shapiro GI, Rollins BJ. p16INK4A as a human tumor suppressor. Biochem Biophys Acta. 1996;1242:165–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

