Different cellular and genetic basis of noise-related endocochlear potential reduction in CBA/J and BALB/cJ mice
- PMID: 20922451
- PMCID: PMC3015030
- DOI: 10.1007/s10162-010-0238-z
Different cellular and genetic basis of noise-related endocochlear potential reduction in CBA/J and BALB/cJ mice
Abstract
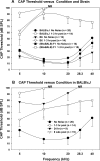
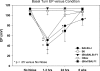
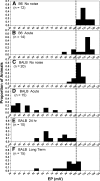
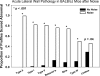
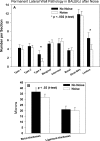
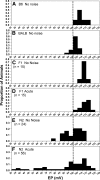
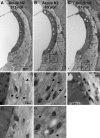
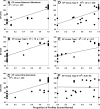
The acute and permanent effects of noise exposure on the endocochlear potential (EP) and cochlear lateral wall were evaluated in BALB/cJ (BALB) inbred mice, and compared with CBA/J (CBA) and C57BL/6 (B6) mice. Two-hour exposure to broadband noise (4-45 kHz) at 110 dB SPL leads to a approximately 50 mV reduction in the EP in BALB and CBA, but not B6. EP reduction in BALB and CBA is reliably associated with characteristic acute cellular pathology in stria vascularis and spiral ligament. By 8 weeks after exposure, the EP in CBA mice has returned to normal. In BALBs, however, the EP remains depressed by an average approximately 10 mV, so that permanent EP reduction contributes to permanent threshold shifts in these mice. We recently showed that the CBA noise phenotype in part reflects the influence of a large effect quantitative trait locus on Chr. 18, termed Nirep (Ohlemiller et al., Hear Res 260:47-53, 2010b). While CBA "EP susceptibility" alleles are dominant to those in B6, examination of (B6 × BALB) F1 hybrid mice and (F1 × BALB) N2 backcross mice revealed that noise-related EP reduction and associated cell pathology in BALBs are inherited in an autosomal recessive manner, and are dependent on multiple genes. Moreover, while N2 mice formed from B6 and CBA retain strong correspondence between acute EP reduction, ligament pathology, and strial pathology, N2s formed from B6 and BALB include subsets that dissociate pathology of ligament and stria. We conclude that the genes and cascades that govern the very similar EP susceptibility phenotypes in BALB and CBA mice need not be the same. BALBs appear to carry alleles that promote more pronounced long term effects of noise on the lateral wall. Separate loci in BALBs may preferentially impact stria versus ligament.
Figures




Similar articles
-
QTL Mapping of Endocochlear Potential Differences between C57BL/6J and BALB/cJ mice.J Assoc Res Otolaryngol. 2016 Jun;17(3):173-94. doi: 10.1007/s10162-016-0558-8. Epub 2016 Mar 15. J Assoc Res Otolaryngol. 2016. PMID: 26980469 Free PMC article.
-
Genetic dependence of cochlear cells and structures injured by noise.Hear Res. 2007 Feb;224(1-2):34-50. doi: 10.1016/j.heares.2006.11.005. Epub 2006 Dec 18. Hear Res. 2007. PMID: 17175124 Free PMC article.
-
A major effect QTL on chromosome 18 for noise injury to the mouse cochlear lateral wall.Hear Res. 2010 Feb;260(1-2):47-53. doi: 10.1016/j.heares.2009.11.006. Epub 2009 Nov 12. Hear Res. 2010. PMID: 19913606 Free PMC article.
-
Mechanisms and genes in human strial presbycusis from animal models.Brain Res. 2009 Jun 24;1277:70-83. doi: 10.1016/j.brainres.2009.02.079. Epub 2009 Mar 12. Brain Res. 2009. PMID: 19285967 Free PMC article. Review.
-
Genetic influences on susceptibility of the auditory system to aging and environmental factors.Scand Audiol Suppl. 1992;36:1-39. Scand Audiol Suppl. 1992. PMID: 1488615 Review.
Cited by
-
Transforming growth factor β1 inhibition protects from noise-induced hearing loss.Front Aging Neurosci. 2015 Mar 20;7:32. doi: 10.3389/fnagi.2015.00032. eCollection 2015. Front Aging Neurosci. 2015. PMID: 25852546 Free PMC article.
-
Divergence of noise vulnerability in cochleae of young CBA/J and CBA/CaJ mice.Hear Res. 2011 Feb;272(1-2):13-20. doi: 10.1016/j.heares.2010.11.006. Epub 2010 Nov 23. Hear Res. 2011. PMID: 21108998 Free PMC article.
-
Identifying microRNAs involved in aging of the lateral wall of the cochlear duct.PLoS One. 2014 Nov 18;9(11):e112857. doi: 10.1371/journal.pone.0112857. eCollection 2014. PLoS One. 2014. PMID: 25405349 Free PMC article.
-
Onset kinetics of noise-induced purinergic adaptation of the 'cochlear amplifier'.Purinergic Signal. 2019 Sep;15(3):343-355. doi: 10.1007/s11302-019-09648-3. Epub 2019 Aug 3. Purinergic Signal. 2019. PMID: 31377959 Free PMC article.
-
OHC-TRECK: A Novel System Using a Mouse Model for Investigation of the Molecular Mechanisms Associated with Outer Hair Cell Death in the Inner Ear.Sci Rep. 2019 Mar 27;9(1):5285. doi: 10.1038/s41598-019-41711-2. Sci Rep. 2019. PMID: 30918314 Free PMC article.
References
-
- Bohne BA, Harding GW, Ou HC. Preparation and evaluation of the mouse temporal bone. In: Willott JF, editor. Handbook of mouse auditory research. Boca Raton, FL: CRC Press; 2001. pp. 171–187.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

