The N-terminus of the intrinsically disordered protein α-synuclein triggers membrane binding and helix folding
- PMID: 20923645
- PMCID: PMC3042581
- DOI: 10.1016/j.bpj.2010.06.035
The N-terminus of the intrinsically disordered protein α-synuclein triggers membrane binding and helix folding
Abstract
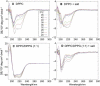
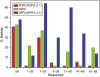
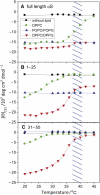
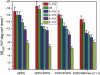
Alpha-synuclein (αS) is a 140-amino-acid protein that is involved in a number of neurodegenerative diseases. In Parkinson's disease, the protein is typically encountered in intracellular, high-molecular-weight aggregates. Although αS is abundant in the presynaptic terminals of the central nervous system, its physiological function is still unknown. There is strong evidence for the membrane affinity of the protein. One hypothesis is that lipid-induced binding and helix folding may modulate the fusion of synaptic vesicles with the presynaptic membrane and the ensuing transmitter release. Here we show that membrane recognition of the N-terminus is essential for the cooperative formation of helical domains in the protein. We used circular dichroism spectroscopy and isothermal titration calorimetry to investigate synthetic peptide fragments from different domains of the full-length αS protein. Site-specific truncation and partial cleavage of the full-length protein were employed to further characterize the structural motifs responsible for helix formation and lipid-protein interaction. Unilamellar vesicles of varying net charge and lipid compositions undergoing lateral phase separation or chain melting phase transitions in the vicinity of physiological temperatures served as model membranes. The results suggest that the membrane-induced helical folding of the first 25 residues may be driven simultaneously by electrostatic attraction and by a change in lipid ordering. Our findings highlight the significance of the αS N-terminus for folding nucleation, and provide a framework for elucidating the role of lipid-induced conformational transitions of the protein within its intracellular milieu.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Beyer K. Mechanistic aspects of Parkinson's disease: α-synuclein and the biomembrane. Cell Biochem. Biophys. 2007;47:285–299. - PubMed
-
- Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. - PubMed
-
- Dev K.K., Hofele K., van der Putten H. Part II: α-synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology. 2003;45:14–44. - PubMed
-
- Moore D.J., West A.B., Dawson T.M. Molecular pathophysiology of Parkinson's disease. Annu. Rev. Neurosci. 2005;28:57–87. - PubMed
-
- Wakabayashi K., Yoshimoto M., Takahashi H. α-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci. Lett. 1998;249:180–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

