Inhibitors of catalase-amyloid interactions protect cells from beta-amyloid-induced oxidative stress and toxicity
- PMID: 20923778
- PMCID: PMC2998107
- DOI: 10.1074/jbc.M110.132860
Inhibitors of catalase-amyloid interactions protect cells from beta-amyloid-induced oxidative stress and toxicity
Abstract
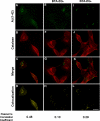
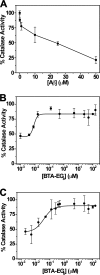
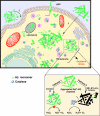
Compelling evidence shows a strong correlation between accumulation of neurotoxic β-amyloid (Aβ) peptides and oxidative stress in the brains of patients afflicted with Alzheimer disease (AD). One hypothesis for this correlation involves the direct and harmful interaction of aggregated Aβ peptides with enzymes responsible for maintaining normal, cellular levels of reactive oxygen species (ROS). Identification of specific, destructive interactions of Aβ peptides with cellular anti-oxidant enzymes would represent an important step toward understanding the pathogenicity of Aβ peptides in AD. This report demonstrates that exposure of human neuroblastoma cells to cytotoxic preparations of aggregated Aβ peptides results in significant intracellular co-localization of Aβ with catalase, an anti-oxidant enzyme responsible for catalyzing the degradation of the ROS intermediate hydrogen peroxide (H(2)O(2)). These catalase-Aβ interactions deactivate catalase, resulting in increased cellular levels of H(2)O(2). Furthermore, small molecule inhibitors of catalase-amyloid interactions protect the hydrogen peroxide-degrading activity of catalase in Aβ-rich environments, leading to reduction of the co-localization of catalase and Aβ in cells, inhibition of Aβ-induced increases in cellular levels of H(2)O(2), and reduction of the toxicity of Aβ peptides. These studies, thus, provide evidence for the important role of intracellular catalase-amyloid interactions in Aβ-induced oxidative stress and propose a novel molecular strategy to inhibit such harmful interactions in AD.
Figures




References
-
- Harman D. (1956) J. Gerontol. 11, 298–300 - PubMed
-
- Subbarao K. V., Richardson J. S., Ang L. C. (1990) J. Neurochem. 55, 342–345 - PubMed
-
- Mecocci P., MacGarvey U., Beal M. F. (1994) Ann. Neurol. 36, 747–751 - PubMed
-
- Hensley K., Hall N., Subramaniam R., Cole P., Harris M., Aksenov M., Aksenova M., Gabbita S. P., Wu J. F., Carney J. M., Lovell M., Markesbery W. R., Butterfield D. A. (1995) J. Neurochem. 65, 2146–2156 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

