Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47
- PMID: 20923780
- PMCID: PMC2998110
- DOI: 10.1074/jbc.M110.172304
Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47
Abstract
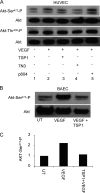
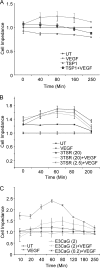
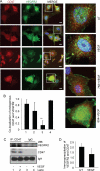
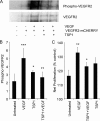
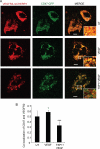
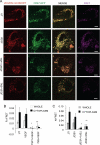
Thrombospondin-1 (TSP1) can inhibit angiogenic responses directly by interacting with VEGF and indirectly by engaging several endothelial cell TSP1 receptors. We now describe a more potent mechanism by which TSP1 inhibits VEGF receptor-2 (VEGFR2) activation through engaging its receptor CD47. CD47 ligation is known to inhibit downstream signaling targets of VEGFR2, including endothelial nitric-oxide synthase and soluble guanylate cyclase, but direct effects on VEGFR2 have not been examined. Based on FRET and co-immunoprecipitation, CD47 constitutively associated with VEGFR2. Ligation of CD47 by TSP1 abolished resonance energy transfer with VEGFR2 and inhibited phosphorylation of VEGFR2 and its downstream target Akt without inhibiting VEGF binding to VEGFR2. The inhibitory activity of TSP1 in large vessel and microvascular endothelial cells was replicated by a recombinant domain of the protein containing its CD47-binding site and by a CD47-binding peptide derived from this domain but not by the CD36-binding domain of TSP1. Inhibition of VEGFR2 phosphorylation was lost when CD47 expression was suppressed in human endothelial cells and in murine CD47-null cells. These results reveal that anti-angiogenic signaling through CD47 is highly redundant and extends beyond inhibition of nitric oxide signaling to global inhibition of VEGFR2 signaling.
Figures
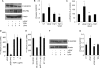
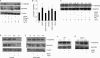
References
-
- Ku D. D., Zaleski J. K., Liu S., Brock T. A. (1993) Am. J. Physiol. 265, H586–H592 - PubMed
-
- Shibuya M. (2006) J. Biochem. Mol. Biol. 39, 469–478 - PubMed
-
- Curwen J. O., Musgrove H. L., Kendrew J., Richmond G. H., Ogilvie D. J., Wedge S. R. (2008) Clin. Cancer Res. 14, 3124–3131 - PubMed
-
- Grothey A., Galanis E. (2009) Nat. Rev. Clin. Oncol. 6, 507–518 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

