Therapeutic potential of mesenchymal stem cells for severe acute lung injury
- PMID: 20923800
- PMCID: PMC2951759
- DOI: 10.1378/chest.10-0518
Therapeutic potential of mesenchymal stem cells for severe acute lung injury
Abstract
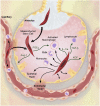
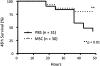
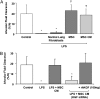
Preclinical studies indicate that allogeneic human mesenchymal stem cells (MSC) may be useful for the treatment of several clinical disorders, including sepsis, acute renal failure, acute myocardial infarction, and more recently, acute lung injury (ALI). This article provides a brief review of the biologic qualities of MSC that make them suitable for the treatment of human diseases, as well as the experimental data that provide support for their potential efficacy for critically ill patients with acute respiratory failure from ALI. The article then discusses which patients with ALI might be the best candidates for cell-based therapy and provides a template for the regulatory and practical steps that will be required to test allogeneic human MSC in patients with severe ALI. There is a dual focus on how to design trials for testing both safety and efficacy.
Figures

References
-
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–247. - PubMed
-
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. - PubMed
-
- Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8(2):110–123. - PubMed
-
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

