In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation
- PMID: 20923880
- PMCID: PMC2964224
- DOI: 10.1073/pnas.1008737107
In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation
Abstract
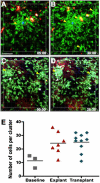
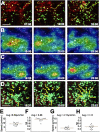
Immune-mediated pulmonary diseases are a significant public health concern. Analysis of leukocyte behavior in the lung is essential for understanding cellular mechanisms that contribute to normal and diseased states. Here, we used two-photon imaging to study neutrophil extravasation from pulmonary vessels and subsequent interstitial migration. We found that the lungs contained a significant pool of tissue-resident neutrophils in the steady state. In response to inflammation produced by bacterial challenge or transplant-mediated, ischemia-reperfusion injury, neutrophils were rapidly recruited from the circulation and patrolled the interstitium and airspaces of the lung. Motile neutrophils often aggregated in dynamic clusters that formed and dispersed over tens of minutes. These clusters were associated with CD115(+) F4/80(+) Ly6C(+) cells that had recently entered the lung. The depletion of blood monocytes with clodronate liposomes reduced neutrophil clustering in the lung, but acted by inhibiting neutrophil transendothelial migration upstream of interstitial migration. Our results suggest that a subset of monocytes serve as key regulators of neutrophil extravasation in the lung and may be an attractive target for the treatment of inflammatory pulmonary diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tsai KS, Grayson MH. Pulmonary defense mechanisms against pneumonia and sepsis. Curr Opin Pulm Med. 2008;14:260–265. - PubMed
-
- Tate MD, et al. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol. 2009;183:7441–7450. - PubMed
-
- Belperio JA, et al. CXCR2/CXCR2 ligand biology during lung transplant ischemia-reperfusion injury. J Immunol. 2005;175:6931–6939. - PubMed
-
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

