The Arabidopsis thylakoid protein PAM68 is required for efficient D1 biogenesis and photosystem II assembly
- PMID: 20923938
- PMCID: PMC2990134
- DOI: 10.1105/tpc.110.077453
The Arabidopsis thylakoid protein PAM68 is required for efficient D1 biogenesis and photosystem II assembly
Abstract
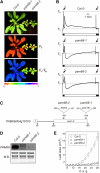
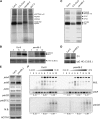
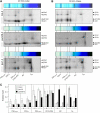
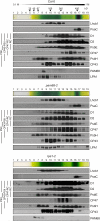
Photosystem II (PSII) is a multiprotein complex that functions as a light-driven water:plastoquinone oxidoreductase in photosynthesis. Assembly of PSII proceeds through a number of distinct intermediate states and requires auxiliary proteins. The photosynthesis affected mutant 68 (pam68) of Arabidopsis thaliana displays drastically altered chlorophyll fluorescence and abnormally low levels of the PSII core subunits D1, D2, CP43, and CP47. We show that these phenotypes result from a specific decrease in the stability and maturation of D1. This is associated with a marked increase in the synthesis of RC (the PSII reaction center-like assembly complex) at the expense of PSII dimers and supercomplexes. PAM68 is a conserved integral membrane protein found in cyanobacterial and eukaryotic thylakoids and interacts in split-ubiquitin assays with several PSII core proteins and known PSII assembly factors. Biochemical analyses of thylakoids from Arabidopsis and Synechocystis sp PCC 6803 suggest that, during PSII assembly, PAM68 proteins associate with an early intermediate complex that might contain D1 and the assembly factor LPA1. Inactivation of cyanobacterial PAM68 destabilizes RC but does not affect larger PSII assembly complexes. Our data imply that PAM68 proteins promote early steps in PSII biogenesis in cyanobacteria and plants, but their inactivation is differently compensated for in the two classes of organisms.
Figures





References
-
- Adir N., Zer H., Shochat S., Ohad I. (2003). Photoinhibition - A historical perspective. Photosynth. Res. 76: 343–370 - PubMed
-
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Aro E.M., Suorsa M., Rokka A., Allahverdiyeva Y., Paakkarinen V., Saleem A., Battchikova N., Rintamäki E. (2005). Dynamics of photosystem II: A proteomic approach to thylakoid protein complexes. J. Exp. Bot. 56: 347–356 - PubMed
-
- Aronsson H., Jarvis P. (2002). A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Lett. 529: 215–220 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

