Exenatide does not evoke pancreatitis and attenuates chemically induced pancreatitis in normal and diabetic rodents
- PMID: 20923958
- PMCID: PMC3006257
- DOI: 10.1152/ajpendo.00479.2010
Exenatide does not evoke pancreatitis and attenuates chemically induced pancreatitis in normal and diabetic rodents
Abstract
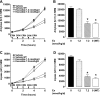
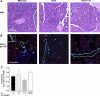
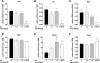
The risk of developing pancreatitis is elevated in type 2 diabetes and obesity. Cases of pancreatitis have been reported in type 2 diabetes patients treated with GLP-1 (GLP-1R) receptor agonists. To examine whether the GLP-1R agonist exenatide potentially induces or modulates pancreatitis, the effect of exenatide was evaluated in normal or diabetic rodents. Normal and diabetic rats received a single exenatide dose (0.072, 0.24, and 0.72 nmol/kg) or vehicle. Diabetic ob/ob or HF-STZ mice were infused with exenatide (1.2 and 7.2 nmol·kg(-1)·day(-1)) or vehicle for 4 wk. Post-exenatide treatment, pancreatitis was induced with caerulein (CRN) or sodium taurocholate (ST), and changes in plasma amylase and lipase were measured. In ob/ob mice, plasma cytokines (IL-1β, IL-2, IL-6, MCP-1, IFNγ, and TNFα) and pancreatitis-associated genes were assessed. Pancreata were weighed and examined histologically. Exenatide treatment alone did not modify plasma amylase or lipase in any models tested. Exenatide attenuated CRN-induced release of amylase and lipase in normal rats and ob/ob mice but did not modify the response to ST infusion. Plasma cytokines and pancreatic weight were unaffected by exenatide. Exenatide upregulated Reg3b but not Il6, Ccl2, Nfkb1, or Vamp8 expression. Histological analysis revealed that the highest doses of exenatide decreased CRN- or ST-induced acute inflammation, vacuolation, and acinar single cell necrosis in mice and rats, respectively. Ductal cell proliferation rates were low and similar across all groups of ob/ob mice. In conclusion, exenatide did not modify plasma amylase and lipase concentrations in rodents without pancreatitis and improved chemically induced pancreatitis in normal and diabetic rodents.
Figures



References
-
- Ahmad SR, Swann J. Exenatide and rare adverse events. N Engl J Med 358: 1970–1971; discussion 1971–1972, 2008 - PubMed
-
- Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol 5: 648–661, 2007 - PubMed
-
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117: 2340–2350, 2008 - PubMed
-
- Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology 5: 132–144, 2005 - PubMed
-
- Blomgren KB, Sundstrom A, Steineck G, Wiholm BE. Obesity and treatment of diabetes with glyburide may both be risk factors for acute pancreatitis. Diabetes Care 25: 298–302, 2002 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

