Aging and bone
- PMID: 20924069
- PMCID: PMC2991386
- DOI: 10.1177/0022034510377791
Aging and bone
Abstract
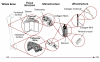
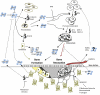
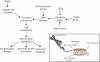
Bones provide mechanical and protective function, while also serving as housing for marrow and a site for regulation of calcium ion homeostasis. The properties of bones do not remain constant with age; rather, they change throughout life, in some cases improving in function, but in others, function deteriorates. Here we review the modifications in the mechanical function and shape of bones, the bone cells, the matrix they produce, and the mineral that is deposited on this matrix, while presenting recent theories about the factors leading to these changes.
Figures
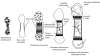






References
-
- Aaron JE, Makins NB, Sagreiya K. (1987). The microanatomy of trabecular bone loss in normal aging men and women. Clin Orthop Relat Res 215:260-271 - PubMed
-
- Adachi T, Aonuma Y, Ito S, Tanaka M, Hojo M, Takano-Yamamoto T, et al. (2009). Osteocyte calcium signaling response to bone matrix deformation. J Biomech 42:2507-2512 - PubMed
-
- Akkus O, Polyakova-Akkus A, Adar F, Schaffler MB. (2003). Aging of microstructural compartments in human compact bone. J Bone Miner Res 18:1012-1019 - PubMed
-
- Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC. (2007b). Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem 282:27298-27305 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

