Epithelial-to-mesenchymal transition promotes tubulin detyrosination and microtentacles that enhance endothelial engagement
- PMID: 20924103
- PMCID: PMC3123454
- DOI: 10.1158/0008-5472.CAN-09-4613
Epithelial-to-mesenchymal transition promotes tubulin detyrosination and microtentacles that enhance endothelial engagement
Abstract
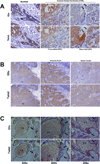
Epithelial-to-mesenchymal transition (EMT) is associated with increased breast tumor metastasis; however, the specific mechanisms by which EMT promotes metastasis remain somewhat unclear. Despite the importance of cytoskeletal dynamics during both EMT and metastasis, very few current studies examine the cytoskeleton of detached and circulating tumor cells. Specific posttranslational α-tubulin modifications are critical for adherent cell motility and implicated in numerous pathologies, but also remain understudied in detached cells. We report here that EMT induced through ectopic expression of Twist or Snail promotes α-tubulin detyrosination and the formation of tubulin-based microtentacles in detached HMLEs. Mechanistically, EMT downregulates the tubulin tyrosine ligase enzyme, resulting in an accumulation of detyrosinated α-tubulin (Glu-tubulin), and increases microtentacles that penetrate endothelial layers to facilitate tumor cell reattachment. Confocal microscopy shows that microtentacles are capable of penetrating the junctions between endothelial cells. Suppression of endogenous Twist in metastatic human breast tumor cells is capable of reducing both tubulin detyrosination and microtentacles. Clinical breast tumor samples display high concordance between Glu-tubulin and Twist expression levels, emphasizing the coupling between EMT and tubulin detyrosination in vivo. Coordinated elevation of Twist and Glu-tubulin at invasive tumor fronts, particularly within ductal carcinoma in situ samples, establishes that EMT-induced tubulin detyrosination occurs at the earliest stages of tumor invasion. These data support a novel model where the EMT that occurs during tumor invasion downregulates tubulin tyrosine ligase, increasing α-tubulin detyrosination and promoting microtentacles that could enhance the reattachment of circulating tumor cells to the vascular endothelium during metastasis.
©2010 AACR.
Figures
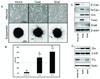



 ) and Snail (
) and Snail ( ) lines attached at significantly faster rates then the HMLE-GFP (
) lines attached at significantly faster rates then the HMLE-GFP ( ), as gauged by electrical impedence expressed as increasing cell index (CI). Lines, mean for 3 triplicate wells; bars, SD; representative graph shown. Three independent experiments were performed (Figure S4). B: CalceinAM-labeled HMLE Twist and Snail cells attach significantly more to confluent human bone marrow endothelial layers (HBME) at 1h than the HMLE-GFP cells (P≤0.005; T-test; black asterisks). Columns, mean for eight experiments; bars, SD. C) Representative images of calcein-AM labeled HMLEs over a confluent HBME layers.
), as gauged by electrical impedence expressed as increasing cell index (CI). Lines, mean for 3 triplicate wells; bars, SD; representative graph shown. Three independent experiments were performed (Figure S4). B: CalceinAM-labeled HMLE Twist and Snail cells attach significantly more to confluent human bone marrow endothelial layers (HBME) at 1h than the HMLE-GFP cells (P≤0.005; T-test; black asterisks). Columns, mean for eight experiments; bars, SD. C) Representative images of calcein-AM labeled HMLEs over a confluent HBME layers.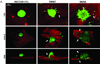
References
-
- Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. - PubMed
-
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

