Drusen characterization with multimodal imaging
- PMID: 20924263
- PMCID: PMC2952278
- DOI: 10.1097/IAE.0b013e3181ee5ce8
Drusen characterization with multimodal imaging
Abstract
Purpose: To characterize the known appearance of cuticular drusen, subretinal drusenoid deposits (reticular pseudodrusen), and soft drusen as revealed by multimodal fundus imaging and to create an explanatory model that accounts for these observations.
Methods: Reported color, fluorescein angiographic, autofluorescence, and spectral domain optical coherence tomography images of patients with cuticular drusen, soft drusen, and subretinal drusenoid deposits were reviewed, as were actual images from affected eyes. Representative histological sections were examined. The geometry, location, and imaging characteristics of these lesions were evaluated. A hypothesis based on the Beer-Lambert law of light absorption was generated to fit these observations.
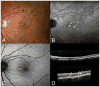
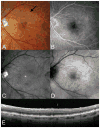
Results: Cuticular drusen appear as numerous, uniform, round, yellow-white punctate accumulations under the retinal pigment epithelium (RPE). Soft drusen are larger, yellow-white dome-shaped mounds of deposit under the RPE. Subretinal drusenoid deposits are polymorphous light-gray interconnected accumulations above the RPE. Based on the model, both cuticular and soft drusen appear yellow because of the removal of shorter wavelength light by a double pass through the RPE. Subretinal drusenoid deposits, which are located on the RPE, are not subjected to short-wavelength attenuation and therefore are more prominent when viewed with blue light. The location and morphology of extracellular material in relationship to the RPE, and associated changes to RPE morphology and pigmentation, appeared to be the primary determinants of druse appearance in different imaging modalities.
Conclusion: Although cuticular drusen, subretinal drusenoid deposits, and soft drusen are composed of common components, they are distinguishable by multimodal imaging because of differences in location, morphology, and optical filtering effects by drusenoid material and the RPE.
Conflict of interest statement
The authors have no financial interests.
Figures













References
-
- Bressler SB, Bressler NM, Sarks SH, Sarks JP. Age-related macular degeneration: nonneovascular early AMD, intermediate AMD, and geographic atrophy. In: Ryan SJ, editor. Retina. Mosby; 2006. pp. 1041–1074.
-
- Klein R, Klein BEK, Linton KLP. Prevalence of age-related maculopathy. Ophthalmol. 1992;99:933–943. - PubMed
-
- van der Schaft TL, Mooy CM, de Bruijn WC, et al. Histologic features of the early stages of age-related macular degeneration. Ophthalmol. 1992;99:278–286. - PubMed
-
- Mullins RF, Hageman GS. Human ocular drusen possess novel core domains with a distinct carbohydrate composition. J Histochem Cytochem. 1999;47:1533–1539. - PubMed
-
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

