A phase II study of weekly docetaxel and cisplatin plus oral tegafur/uracil and leucovorin as first-line chemotherapy in patients with locally advanced or metastatic gastric cancer
- PMID: 20924378
- PMCID: PMC2990611
- DOI: 10.1038/sj.bjc.6605928
A phase II study of weekly docetaxel and cisplatin plus oral tegafur/uracil and leucovorin as first-line chemotherapy in patients with locally advanced or metastatic gastric cancer
Abstract
Background: Docetaxel plus cisplatin and 5-fluorouracil has become a new standard for treating advanced gastric cancer. However, high rates of severe neutropenia limit its application. Modification of the regimen could be the solution to get similar activity but less myelosuppression.
Methods: Patients with histologically confirmed, locally advanced, or recurrent/metastatic gastric adenocarcinoma without previous chemotherapy were enrolled. This regimen consisted of docetaxel (Tyxan, TTY, Taipei, Taiwan) 30-min infusion at a dose of 36 mg m(-2), followed by cisplatin 30 mg m(-2) infusion over 1 h on days 1 and 8, and oral tegafur/uracil 300 mg m(-2) per day plus leucovorin 90 mg per day on days 1-14, every 3 weeks. Tumour response was evaluated after every 2 cycles of treatment.
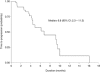
Results: From August 2007 to March 2009, 45 patients were enrolled. The median age was 56 years (range: 22-75). Among the 40 patients evaluable for tumour response, one achieved a complete response, 22 had partial responses and 11 had stable disease. The overall response rates of the evaluable and intent-to-treat (ITT) populations were 58% (95% CI: 41-74%) and 53% (95% CI: 38-68%), respectively. The disease control rates in these populations were 85% (95% CI: 70-94%) and 82% (95% CI: 68-92%), respectively. In the ITT analysis, the median time to progression and overall survival were 6.8 and 13.9 months, respectively. Major grade 3-4 toxicities were neutropenia (51%), anaemia (22%), diarrhoea (16%), and infections (20%). No patient died of treatment-related toxicities.
Conclusion: Concurrent weekly docetaxel and cisplatin plus oral tegafur/uracil and leucovorin are effective and well tolerated in the treatment of advanced gastric cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, Majlis A, Assadourian S, Van Cutsem E (2005) Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol 23: 5660–5667 - PubMed
-
- Chen LT, Liu TW, Wu CW, Chung TR, Shiah HS, Jan CM, Liu JM, Whang-Peng J, Chang JY (2002) A phase I study of weekly docetaxel, 24-h infusion of high-dose fluorouracil/leucovorin and cisplatin in patients with advanced gastric cancer. Oncology 63: 239–247 - PubMed
-
- Chong G, Cunningham D (2005) Gastrointestinal cancer: recent developments in medical oncology. Eur J Surg Oncol 31: 453–460 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical