Detecting treatment response in a model of human breast adenocarcinoma using hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate
- PMID: 20924379
- PMCID: PMC2990617
- DOI: 10.1038/sj.bjc.6605945
Detecting treatment response in a model of human breast adenocarcinoma using hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate
Abstract
Background: The recent introduction of a dynamic nuclear polarisation technique has permitted noninvasive imaging of tumour cell metabolism in vivo following intravenous administration of (13)C-labelled cell substrates.
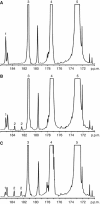
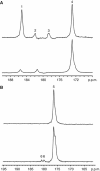
Methods: Changes in hyperpolarised [1-(13)C]pyruvate and [1,4-(13)C(2)]fumarate metabolism were evaluated in both MDA-MB-231 cells and in implanted MDA-MB-231 tumours following doxorubicin treatment.
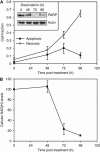
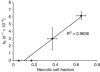
Results: Treatment of MDA-MB-231 cells resulted in the induction of apoptosis, which was accompanied by a decrease in hyperpolarised (13)C label flux between [1-(13)C]pyruvate and lactate, which was correlated with a decrease in the cellular NAD(H) coenzyme pool. There was also an increase in the rate of fumarate conversion to malate, which accompanied the onset of cellular necrosis. In vivo, the decrease in (13)C label exchange between pyruvate and lactate and the increased flux between fumarate and malate, following drug treatment, were shown to occur in the absence of any detectable change in tumour size.
Conclusion: We show here that the early responses of a human breast adenocarcinoma tumour model to drug treatment can be followed by administration of both hyperpolarised [1-(13)C]pyruvate and [1,4-(13)C(2)]fumarate. These techniques could be used, therefore, in the clinic to detect the early responses of breast tumours to treatment.
Figures

References
-
- Aboagye EO, Bhujwalla ZM (1999) Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res 59(1): 80–84 - PubMed
-
- Aubin JE (1979) Autofluorescence of viable cultured mammalian cells. J Histochem Cytochem 27(1): 36–43 - PubMed
-
- Biersack HJ, Bender H, Palmedo H (2004) FDG-PET in monitoring therapy of breast cancer. Eur J Nucl Med Mol Imaging 31(Suppl 1): S112–S117 - PubMed
-
- Brindle K (2008a) New approaches for imaging tumour responses to treatment. Nat Rev Cancer 8(2): 94–107 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

