Sox17 and chordin are required for formation of Kupffer's vesicle and left-right asymmetry determination in zebrafish
- PMID: 20925124
- PMCID: PMC3090657
- DOI: 10.1002/dvdy.22431
Sox17 and chordin are required for formation of Kupffer's vesicle and left-right asymmetry determination in zebrafish
Abstract
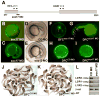
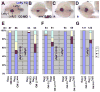
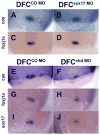
Kupffer's vesicle (KV), a ciliated fluid-filled sphere in the zebrafish embryo with a critical role in laterality determination, is derived from a group of superficial cells in the organizer region of the gastrula named the dorsal forerunner cells (DFC). We have examined the role of the expression of sox17 and chordin (chd) in the DFC in KV formation and laterality determination. Whereas sox17 was known to be expressed in DFC, its function in these cells was not studied before. Further, expression of chd in these cells has not been reported previously. Targeted knockdown of Sox17 and Chd in DFC led to aberrant Left-Right (L-R) asymmetry establishment, as visualized by the expression of southpaw and lefty, and heart and pancreas placement in the embryo. These defects correlated with the formation of small KVs with apparently diminished cilia, consistent with the known requirement for ciliary function in the laterality organ for the establishment of L-R asymmetry.
Published 2010 Wiley-Liss, Inc.
Figures




References
-
- Alexander J, Rothenberg M, Henry GL, Stainier DY. casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol. 1999;215:343–357. - PubMed
-
- Amack JD, Wang X, Yost HJ. Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer's vesicle in zebrafish. Dev Biol. 2007;310:196–210. - PubMed
-
- Amack JD, Yost HJ. The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr Biol. 2004;14:685–690. - PubMed
-
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

