Partitioning and localization of environment-sensitive 2-(2'-pyridyl)- and 2-(2'-pyrimidyl)-indoles in lipid membranes: a joint refinement using fluorescence measurements and molecular dynamics simulations
- PMID: 20925327
- PMCID: PMC4470561
- DOI: 10.1021/jp106981c
Partitioning and localization of environment-sensitive 2-(2'-pyridyl)- and 2-(2'-pyrimidyl)-indoles in lipid membranes: a joint refinement using fluorescence measurements and molecular dynamics simulations
Abstract
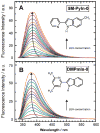
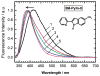
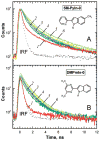
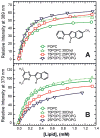
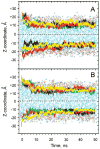
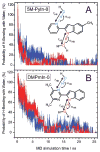
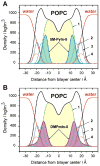
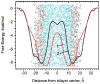
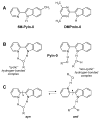
Fluorescence of environment-sensitive dyes is widely applied to monitor local structure and solvation dynamics of biomolecules. It has been shown that, in comparison with a parent indole fluorophore, fluorescence of 2-(2'-pyridyl)-5-methylindole (5M-PyIn-0) and 2-[2'-(4',6'-dimethylpyrimidyl)]-indole (DMPmIn-0) is remarkably sensitive to hydrogen bonding with protic partners. Strong fluorescence, observed for these compounds in nonpolar and polar aprotic solvents, is efficiently quenched in aqueous solution. This study demonstrates that 5M-PyIn-0 and DMPmIn-0, which are almost nonemitting in aqueous solution, become highly fluorescent upon titrating with phospholipid vesicles. The fluorescence enhancement is accompanied by a significant blue shift of emission maximum. The Gibbs free energy of membrane partitioning, measured by the increase in the steady-state fluorescence intensities during transfer from an aqueous environment to a lipid bilayer, is very favorable for both compounds, being in a range from -7.1 to -8.0 kcal/mol and depending only slightly on lipid composition of the membrane. The fluorescence enhancement upon membrane partitioning is indicative of the loss of the specific hydrogen-bonding interactions between the excited fluorophore and water molecules, causing efficient fluorescence quenching in bulk water. This conclusion is supported by atomistic molecular dynamics (MD) simulations, demonstrating that both 5M-PyIn-0 and DMPmIn-0 bind rapidly and partition deeply into a lipid bilayer. MD simulations also show a rapid, nanosecond-scale decrease in the probability of solute-solvent hydrogen bonding during passive diffusion of the probe molecules from bulk water into a lipid bilayer. At equilibrium conditions, both 5M-PyIn-0 and DMPmIn-0 prefer deep localization within the hydrophobic, water-free region of the bilayer. A free energy profile of penetration across a bilayer estimated using MD umbrella sampling shows that both indole derivatives favor residence in a rather wide potential energy well located 10-15 Å from the bilayer center.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

