Dystrophin immunity in Duchenne's muscular dystrophy
- PMID: 20925545
- PMCID: PMC3014106
- DOI: 10.1056/NEJMoa1000228
Dystrophin immunity in Duchenne's muscular dystrophy
Abstract
We report on delivery of a functional dystrophin transgene to skeletal muscle in six patients with Duchenne's muscular dystrophy. Dystrophin-specific T cells were detected after treatment, providing evidence of transgene expression even when the functional protein was not visualized in skeletal muscle. Circulating dystrophin-specific T cells were unexpectedly detected in two patients before vector treatment. Revertant dystrophin fibers, which expressed functional, truncated dystrophin from the deleted endogenous gene after spontaneous in-frame splicing, contained epitopes targeted by the autoreactive T cells. The potential for T-cell immunity to self and nonself dystrophin epitopes should be considered in designing and monitoring experimental therapies for this disease. (Funded by the Muscular Dystrophy Association and others; ClinicalTrials.gov number, NCT00428935.).
Figures

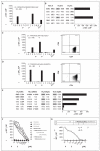

Comment in
-
Autoimmunity in a genetic disease—a cautionary tale.N Engl J Med. 2010 Oct 7;363(15):1473-5. doi: 10.1056/NEJMe1009056. N Engl J Med. 2010. PMID: 20925551 No abstract available.
-
Dystrophin Immunity after Gene Therapy for Duchenne's Muscular Dystrophy.N Engl J Med. 2023 Jun 15;388(24):2294-2296. doi: 10.1056/NEJMc2212912. N Engl J Med. 2023. PMID: 37314712 No abstract available.
References
-
- Emery AE. The muscular dystrophies. Lancet. 2002;359:687–95. - PubMed
-
- Fabb SA, Wells DJ, Serpente P, Dickson G. Adeno-associated virus vector gene transfer and sarcolemmal expression of a 144 kDa micro-dystrophin effectively restores the dystrophin-associated protein complex and inhibits myofibre degeneration in nude/mdx mice. Hum Mol Genet. 2002;11:733–41. - PubMed
-
- Mendell JR, Moxley RT, Griggs RC, et al. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med. 1989;320:1592–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
